Classification of Polymers
Category : JEE Main & Advanced
(1) Classification based on source of availability : They are classified as
(i) Natural polymers (ii) Synthetic polymers (iii) Semi-synthetic polymers
(i) Natural polymers : The polymers obtained from nature (plants and animals) are called natural polymers. These polymers are very essential for life. They are as under.
(a) Starch : It is polymer of glucose and it is food reserve of plant.
(b) Cellulose : It is also a polymer of glucose. It is a chief structural material of the plant both starch and cellulose are made by plants from glucose produced during photosynthesis.
(c) Proteins : These are polymers of a-amino acids, they have generally 20 to 1000 \[\alpha \] amino acid joined together in a highly organized arrangement. These are building blocks of animal body and constitute an essential part of our food.
(d) Nucleic acids : These are polymers of various nucleotides. For example RNA and DNA are common nucleotides.
(ii) Synthetic polymers : The polymers which are prepared in the laboratories are called synthetic polymers. These are also called man made polymers. For example polyethene, PVC nylon, teflon, bakelite terylene, synthetic rubber etc.
(iii) Semisynthetic polymers : These polymers are mostly derived from naturally occurring polymers by chemical modifications. For example cellulose is naturally occurring polymers, cellulose on acetylation with acetic anhydride in the presence of sulphuric acid forms cellulose diacetate polymers. It is used in making thread and materials like films glasses etc. Vulcanized rubber is also an example of semisynthetic polymers used in making tyres etc. gun cotton which is cellulose nitrate used in making explosive.
(2) Classification based upon structure : On the basis of structure of polymers these can be classified as
(i) Linear polymers
(ii) Branched chain polymers
(iii) Cross linked polymers
(i) Linear polymers : These are polymers in which monomeric units are linked together to form linear chain. These linear polymers are well packed and have high magnitude of intermolecular forces of attraction and therefore have high densities, high tensil (pulling) strength and high melting points. Some common example of linear polymers are high density polyethylene nylon, polyester, PVC, PAN etc.
(ii) Branched chain polymers : These are polymers in which the monomers are joined to form long chains with side chains or branches of different lengths. These branched chain polymers are irregularly packed and therefore, they have low tensile strength, low density, boiling point and melting points than linear polymers. Some common examples are low density polythene, glycogen, starch etc. (Amylopectin).
(iii) Cross linked polymers : These are polymers in which monomers unit are crosslinked together to form a three dimensional network polymers. These polymers are hard, rigid and brittle because of network structure e.g., Bakelite, malamine formaldehyde resin etc.
(3) Classification based upon molecular forces : Depending upon the intermolecular forces, the polymers have been classified into four type.
(i) Elastomers
(ii) Fibres
(iii) Thermoplastics
(iv) Thermosetting polymers
(i) Elastomers : The polymers that have elastic character like rubber (a material that can return to its original shape after stretching is said to be elastic) are called elastomers. In elastomers the polymers chains are held together by weak intermolecular forces. Because of the presence of weak forces, the polymers can be easily stretched by applying small stress and regains their original shape when the stress is removed. The most important example of elastomers is natural rubber.
(ii) Fibres : These are the polymers which have strong intermolecular forces between the chain. These forces are either hydrogen bonds or dipole-dipole interaction. Because of strong forces, the chains are closely packed giving them high tensil strength and less elasticity. Therefore, these polymers have sharp melting points. These polymers are long, thin and thread like and can be woven in fabric. Therefore, these are used for making fibres.
Example : Nylon 66, dacron, silk etc.
(iii) Thermoplastics : These are the polymers which can be easily softened repeatedly when heated and hardened when cooled with little change in their properties. The intermolecular forces in these polymers are intermediate between those of elastomers and fibres. There is no cross linking between the chain. The softening occurs as the polymer chain move more and more freely because of absence of cross link. When heated, they melt and form a fluid which can be moulded into any desired shapes and then cooled to get the desired product.
Example : Polythene, polystyrene, PVC, teflon etc.
(iv) Thermosetting polymers : These are the polymers which undergo permanent change on heating. They become hard and infusible on heating. They are generally prepared from low molecular mass semifluid substances. When heated they get highly cross linked to form hard infusible and insoluble products. The cross links hold the molecule in place so that heating does not allow them to move freely. Therefore a thermosetting plastic is cross linked and is permanently rigid.
Example : Bakelite, melamine formaldehyde resin etc.
Difference between thermoplastic and thermosetting polymers
| Thermoplastic polymers | Thermosetting polymers |
| (1) These soften and melt on heating. | These do not soften on heating but rather become hard in case prolonged heating is done these start burning. |
| (2) These can be remoulded recast and reshaped. | These can not be remoulded or reshaped. |
| (3) These are less brittle and soluble in some organic solvents. | These are more brittle and insoluble in organic solvents. |
| (4) These are formed by addition polymerisation. | These are formed by condensation polymerisation. |
| (5) These have usually linear structures. Ex. Polyethylene, PVC, teflon. | These have three dimensional cross linked structures. Ex. Bakelite, urea, formaldehyde, resin. |
(4) Classification based upon mode of synthesis : They are of two types on the basis of their synthesis.
(i) Addition polymers
(ii) Condensation polymers
(i) Addition polymers : A polymer formed by direct addition of repeated monomers without the elimination of by product molecule is called addition polymers. For example,
\[\underset{\text{Ethene}}{\mathop{nC{{H}_{2}}=C{{H}_{2}}}}\,\to \underset{\text{Polyethene}}{\mathop{{{(-C{{H}_{2}}-C{{H}_{2}}-)}_{n}}}}\,\]
\[\underset{\text{Propylene}}{\mathop{nC{{H}_{3}}-CH=C{{H}_{2}}}}\,\to \underset{\text{Polypropylene}}{\mathop{{{\left[ -C{{H}_{2}}-\underset{C{{H}_{3}}\,\,}{\mathop{\underset{|}{\mathop{C}}\,H-}}\, \right]}_{n}}}}\,\]
(ii) Condensation polymers : A polymer formed by the condensation of two or more than two monomers with the elimination of simple molecule like water, ammonia, HCl, alcohol etc. is called condensation polymers. For example,
\[\underset{\text{Hexamethylenediamine}}{\mathop{n{{H}_{2}}N-{{(C{{H}_{2}})}_{6}}-N{{H}_{2}}}}\,+\underset{\text{Adipic acid}}{\mathop{nHOOC-{{(C{{H}_{2}})}_{4}}-COOH}}\,\]\[\xrightarrow{-n{{H}_{2}}O}{{\left( \underset{\text{Nylon-66}}{\mathop{-NH-{{(C{{H}_{2}})}_{6}}-NH-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-{{(C{{H}_{2}})}_{4}}-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-}}\, \right)}_{n}}\]
Difference between addition and condensation polymers
| Addition polymers | Condensation polymers |
| Formed by addition reaction. | Formed by condensation process with elimination of small molecules like \[{{H}_{2}}O\]. |
| Molecular mass is a whole number multiple of the monomer. | Molecular mass is not whole number multiple of the monomer units. |
| Generally involve one monomer unit. | Generally involve more than one monomer unit. |
| Monomers are unsaturated molecules. | Monomer units must have two active functional groups. |
| They are generally chain growth polymers. | They are generally step growth polymers. |
(5) Classification based upon the nature of monomer : On the basis of nature of monomer. Polymer are of two types
(i) Homopolymers
(ii) Copolymers
(i) Homopolymers : A polymer formed from one type of monomers is called homopolymer. For example, polythene is a homopolymer of monomer ethene.
\[\underset{\text{Ethene}}{\mathop{n{{H}_{2}}C=C{{H}_{2}}}}\,\xrightarrow{\text{Polymerisation}}{{(-\underset{\text{Polythene}}{\mathop{C{{H}_{2}}-C{{H}_{2}}-}}\,)}_{n}}\text{ Homopolymer}\]
(ii) Copolymers : A polymer formed from two or more different monomers is called copolymer or mixed polymer. For example, nylon-66 is a polymer of two types of monomers : hexamethylenediamine and adipic acid.
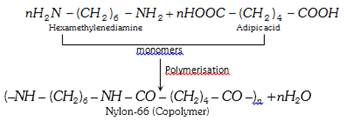
Copolymer are commercially more important.
For example copolymerisation of vinyl chloride with vinylidene chloride (1, 1 dichloroethane) in a 1 : 4 ratio forms a copolymer known as saran.
\[\underset{\text{Vinyl chloride}}{\mathop{m{{H}_{2}}C=\underset{Cl\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,}}\,\,+\underset{\text{Vinylidene chloride}}{\mathop{nC{{H}_{2}}=C\,C{{l}_{2}}}}\,\xrightarrow{\text{Polymerisation}}\]\[\underset{\text{Saran polymer}}{\mathop{{{\left( -C{{H}_{2}}-\overset{Cl\,\,}{\mathop{\overset{|}{\mathop{C}}\,H}}\, \right)}_{m}}}}\,{{\left( C{{H}_{2}}-\underset{Cl\,}{\overset{Cl\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-}}}\, \right)}_{n}}\]
Copolymerisation of monomer mixtures often leads to the formation of polymers which have quite different properties than those of either corresponding homopolymer. For example, a mixture of styrene and methyl methacrylate can form a copolymer.
\[\underset{\text{Styrene}}{\mathop{C{{H}_{2}}=\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{C}}\,H\,\,\,}}\,}}\,+\underset{\begin{smallmatrix} \text{Methyl} \\ \text{methacrylate} \end{smallmatrix}}{\mathop{{{H}_{2}}C=\underset{COOC{{H}_{3}}\,\,\,\,\,\,\,\,\,}{\overset{C{{H}_{3}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,\to \,\,\tilde{\ }C{{H}_{2}}-}}}\,}}\,\underset{\text{Copolymer}}{\mathop{\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{C}}\,H-}}\,C{{H}_{2}}-\underset{COOC{{H}_{3}}}{\overset{C{{H}_{3}}\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,\tilde{\ }\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
The composition of the copolymer depends on the proportion of the monomers and their reactivity. It may be noted that some monomers do not polymerise at all but copolymerize. For example, maleic anhydride does not polymerise as such. However, it copolymerises with styrene in a highly symmetrical manner to form styrene maleic anhydride copolymer.
It may be noted that many types of copolymers can be obtained depending upon the distribution of monomer units in the chain. Two monomers can combine in either regular fashion (although this is rare) or random fashion. For example, if monomer A is copolymerised with monomer B, the resultant product may have a random distribution of the two units throughout the chain or it might have alternating distribution.
\[(\text{ }A\text{ }\text{ }B\text{ }\text{ }A\text{ }\text{ }B\text{ }\text{ }A\text{ }\text{ }B\text{ }\text{ }A\text{ }\text{ }B\text{ })\] Alternating copolymer
\[(\text{ }A\text{ }\text{ }A\text{ }\text{ }A\text{ }\text{ }B\text{ }\text{ }A\text{ }\text{ }B\text{ }\text{ }B\text{ }\text{ }A\text{ }\text{ }B\text{ })\] Random copolymer
The exact distribution of monomer units depends on the initial proportion of the two-reactant monomers and their reactivities. Most copolymers have varying distributions. Two other types of copolymers that can be prepared under certain conditions are called block copolymers and graft copolymers.
(a) Block copolymers are those in which different blocks of identical monomer units alternate with each other as
\[{{(AAAABBBBAAAABBBB)}_{n}}\]
These are prepared by initiating the polymerisation of one monomer as if growing a homopolymer and then adding an excess of second monomer to the active reaction mixture.
(b) Graft polymers are those in which homopolymer branches of one monomer units are grafted on the homopolymer chains of another monomer units as :
\[{{(-A-\underset{{{|}^{n}}}{\mathop{\underset{B}{\mathop{\underset{|}{\mathop{\underset{B}{\mathop{\underset{|}{\mathop{\underset{B}{\mathop{\underset{|}{\mathop{A}}\,}}\,}}\,}}\,}}\,}}\,}}\,-A-A-A-A-\underset{{{|}^{n}}}{\mathop{\underset{B}{\mathop{\underset{|}{\mathop{\underset{B}{\mathop{\underset{|}{\mathop{\underset{B}{\mathop{\underset{|}{\mathop{A}}\,}}\,}}\,}}\,}}\,}}\,}}\,-A-A-A-\underset{{{|}^{n}}}{\mathop{\underset{B}{\mathop{\underset{|}{\mathop{\underset{B}{\mathop{\underset{|}{\mathop{\underset{B}{\mathop{\underset{|}{\mathop{A}}\,}}\,}}\,}}\,}}\,}}\,}}\,-A-)}_{n}}\]
These are prepared by radiation of \[\gamma \]-rays on a completed homopolymer chain in the presence of the second monomer. The high energy radiation knocks hydrogen atoms of the homopolymer chain at random points resulting radical sites for initiation of the added monomer. By careful control of the polymerisation reaction, we can produce copolymers of desired properties by combination of different monomers in various ratios and geometric arrangements.
You need to login to perform this action.
You will be redirected in
3 sec
