Alkaline Earth Metals and Their Compounds
Category : JEE Main & Advanced
The group 2 of the periodic table consists of six metallic elements. These are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra). These (except Be) are known as alkaline earth metals as their oxides are alkaline and occur in earth crust.
(1) Electronic configuration
| Element | Electronic configurations (\[n{{s}^{2}}\]) |
| \[_{4}Be\] | \[[He]\,2{{s}^{2}}\] |
| \[_{12}Mg\] | \[[Ne]\,3{{s}^{2}}\] |
| \[_{20}Ca\] | \[[Ar]\,4{{s}^{2}}\] |
| \[_{38}Sr\] | \[[Kr]\,5{{s}^{2}}\] |
| \[_{56}Ba\] | \[[Xe]\,6{{s}^{2}}\] |
| \[_{88}Ra\] | \[[Rn]\,7{{s}^{2}}\] |
Radium was discovered in the ore pitch blende by madam Curie. It is radioactive in nature.
(2) Occurrence : These are found mainly in combined state such as oxides, carbonates and sulphates Mg and Ca are found in abundance in nature. Be is not very abundant, Sr and Ba are less abundant. Ra is rare element. Some important ores of alkaline earth metals are given below,
(i) Baryllium : Beryl (3BeO.Al2O3.6SiO2); Phenacite (Be2SiO4)
(ii) Magnesium : Magnesite (MgCO3); Dolomite (CaCO3. MgCO3); Epsomite(MgSO4. 7H2O); Carnallite (MgCl2.KCl. 6H2O); Asbestos [CaMg3(SiO3)4]
(iii) Calcium : Limestone (CaCO3); Gypsum : (CaSO4.2H2O), Anhydrite (CaSO4); Fluorapatite [(3Ca3(PO4)2.CaF2)] Phosphorite rock [Ca3(PO4)2]
(iv) Barium : Barytes (BaSO4) ; witherite (BaCO3)
(v) Radium : Pitch blende (U3O8); (Ra in traces); other radium rich minerals are carnotite [K2UO2)] (VO4)2 8H2O and antamite [Ca(UO2)2]
(3) Extraction of alkaline earth metals
(i) Be and Mg are obtained by reducing their oxides carbon,
BeO + C \[\to \] Be + CO ; MgO + C \[\to \] Mg + CO
(ii) The extraction of alkaline earth metals can also be made by the reduction of their oxides by alkali metals or by electrolysing their fused salts.
(4) Alloy formation : These dissolve in mercury and form amalgams.
Physical properties
(1) Physical state : All are greyish-white, light, malleable and ductile metals with metallic lustre. Their hardness progressively decrease with increase in atomic number. Although these are fairly soft but relatively harder than alkali metals.
(2) Atomic and ionic radii
(i) The atomic and ionic radii of alkaline earth metals also increase down the group due to progressive addition of new energy shells like alkali metals.
| Be | Mg | Ca | Sr | Ba | Ra | |
| Atomic radius (pm) | 112 | 160 | 197 | 215 | 222 | – |
| Ionic radius of M2+ ion (pm) | 31 | 65 | 99 | 113 | 135 | 140 |
(ii) The atomic radii of alkaline earth metals are however smaller than their corresponding alkali metal of the same period. This is due to the fact that alkaline earth metals possess a higher nuclear charge than alkali metals which more effectively pulls the orbit electrons towards the nucleus causing a decrease in size.
(3) Density
(i) Density decreases slightly upto Ca after which it increases. The decrease in density from Be to Ca might be due to less packing of atoms in solid lattice of Mg and Ca.
| Be | Mg | Ca | Sr | Ba | Ra |
| 1.84 | 1.74 | 1.55 | 2.54 | 3.75 | 6.00 |
(ii) The alkaline earth metals are more denser, heavier and harder than alkali metal. The higher density of alkaline earth metals is due to their smaller atomic size and strong intermetallic bonds which provide a more close packing in crystal lattice as compared to alkali metals.
(4) Melting point and Boiling point
(i) Melting points and boiling points of alkaline earth metals do not show any regular trend.
| Be | Mg | Ca | Sr | Ba | Ra | |
| melting points (K) | 1560 | 920 | 1112 | 1041 | 1000 | 973 |
| boiling point (K) | 2770 | 1378 | 1767 | 1654 | 1413 | – |
(ii) The values are, however, more than alkali metals. This might due to close packing of atoms in crystal lattice in alkaline earth metals.
(5) Ionisation energy and electropositive or metallic character
(i) Since the atomic size decreases along the period and the nuclear charge increases and thus the electrons are more tightly held towards nucleus. It is therefore alkaline earth metals have higher ionisation energy in comparison to alkali metals but lower ionisation energies in comparison to p-block elements.
(ii) The ionisation energy of alkaline earth metals decreases from Be to Ba.
| Be | Mg | Ca | Sr | Ba | Ra | |
| First ionisation energy (k J mol-1) | 899 | 737 | 590 | 549 | 503 | 509 |
| Second ionisation energy (kJ mol-1) | 1757 | 1450 | 1146 | 1064 | 965 | 979 |
(iii) The higher values of second ionisation energy is due to the fact that removal of one electron from the valence shell, the remaining electrons are more tightly held in which nucleus of cation and thus more energy is required to pull one more electron from monovalent cation.
(iv) No doubt first ionisation energy of alkaline earth metals are higher than alkali metals but a closer look on 2nd ionisation energy of alkali metals and alkaline earth metals reveals that 2nd ionisation energy of alkali metals are more
| Li | Be | |
| 1st ionisation energy (kJ mol–1) | 520 | 899 |
| 2nd ionisation energy (kJ mol–1) | 7296 | 1757 |
This may be explained as,
\[Li:\,1{{s}^{2}},\,\,2{{s}^{1}}\underset{\text{electron}}{\mathop{\xrightarrow{\text{removal}\,\text{of}\,2s}}}\,L{{i}^{+}}:1{{s}^{2}}\underset{\text{electron}}{\mathop{\xrightarrow{\text{removal}\,\text{of}\,1s}}}\,\,L{{i}^{2+}}:1{{s}^{1}}\]
Be : 1s2 , 2s2 \[\underset{electron}{\mathop{\xrightarrow{removal\,of\,2s}}}\,\] Be+ : 1s2, 2s1 \[\underset{electron}{\mathop{\xrightarrow{removal\,of\,2s}}}\,\]Be2+ : 1s2
The removal of 2nd electron from alkali metals takes place from 1s sub shell which are more closer to nucleus and exert more nuclear charge to hold up 1s electron core, whereas removal of 2nd electron from alkaline earth metals takes from 2s sub shell. More closer are shells to the nucleus, more tightly are held electrons with nucleus and thus more energy is required to remove the electron.
(v) All these possess strong electropositive character which increases from Be to Ba.
(vi) These have less electropositive character than alkali metals as the later have low values of ionisation energy.
(6) Oxidation number and valency
(i) The IE1 of the these metals are much lower than IE1 and thus it appears that these metals should form univalent ion rather than divalent ions but in actual practice, all these give bivalent ions. This is due to the fact that M2+ ion possesses a higher degree of hydration or M2+ ions are extensively hydrated to form [M(H2O)x]2+, a hydrated ion. This involves a large amount of energy evolution which counter balances the higher value of second ionisation energy.
\[M\to {{M}^{2+}},\,\,\Delta H=I{{E}_{1}}+{{E}_{2}}\]
\[{{M}^{2+}}{{+}_{x}}{{H}_{2}}O\to {{[M{{({{H}_{2}}O)}_{x}}]}^{2+}};\,\Delta H=-\] hydration energy.
(ii) The tendency of these metals to exist as divalent cation can thus be accounted as,
(a) Divalent cation of these metals possess noble gas or stable configuration.
(b) The formation of divalent cation lattice leads to evolution of energy due to strong lattice structure of divalent cation which easily compensates for the higher values of second ionisation energy of these metals.
(c) The higher heats of hydration of divalent cation which accounts for the existence of the divalent ions of these metals in solution state.
(7) Hydration of ions
(i) The hydration energies of alkaline earth metals divalent cation are much more than the hydration energy of monovalent cation.
| \[M{{g}^{+}}\] | \[M{{g}^{2+}}\] | |
| Hydration energy or Heat of hydration (kJ mol–1) | 353 | 1906 |
The abnormally higher values of heat of hydration for divalent cations of alkaline earth metals are responsible for their divalent nature. MgCl2 formation occurs with more amount of heat evolution and thus MgCl2 is more stable.
(ii) The hydration energies of M2+ ion decreases with increase in ionic radii.
| \[B{{e}^{2+}}\] | \[M{{g}^{2+}}\] | \[C{{a}^{2+}}\] | \[S{{r}^{2+}}\] | \[B{{a}^{2+}}\] | |
| Heat of hydration kJ mol–1 | 2382 | 1906 | 1651 | 1484 | 1275 |
(iii) Heat of hydration are larger than alkali metals ions and thus alkaline earth metals compounds are more extensively hydrated than those of alkali metals e.g MgCl2 and CaCl2 exists as Mg Cl2 .6H2O and CaCl2. 6H2O which NaCl and KCl do not form such hydrates.
(iv) The ionic mobility, therefore, increases from Be2+ to Ba2+, as the size of hydrated ion decreases.
(8) Electronegativities
(i) The electronegativities of alkaline earth metals are also small but are higher than alkali metals.
(ii) Electronegativity decreases from Be to Ba as shown below,
| Be | Mg | Ca | Sr | Ba | |
| Electronegativity | 1.57 | 1.31 | 1.00 | 0.95 | 0.89 |
(9) Conduction power : Good conductor of heat and electricity.
(10) Standard oxidation potential and reducing properties
(i) The standard oxidation potential (in volts) are,
| Be | Mg | Ca | Sr | Ba |
| 1.69 | 2.35 | 2.87 | 2.89 | 2.90 |
(ii) All these metals possess tendency to lose two electrons to give M2+ ion and are used as reducing agent.
(iii) The reducing character increases from Be to Ba, however, these are less powerful reducing agent than alkali metals.
(iv) Beryllium having relatively lower oxidation potential and thus does not liberate H2 from acids.
(11) Characteristic flame colours
The characteristic flame colour shown are : Ca - brick red; Sr –crimson ; Ba-apple green and Ra- crimson.
Chemical Properties
(1) Formation of oxides and hydroxides
(i) The elements (except Ba and Ra) when burnt in air give oxides of ionic nature M2+O2- which are crystalline in nature. Ba and Ra however give peroxide. The tendency to form higher oxides increases from Be to Ra.
2M + O2 \[\to \] 2MO (M is Be, Mg or Ca )
2M + O2 \[\to \] MO2 (M is Ba or Sr)
(ii) Their less reactivity than the alkali metals is evident by the fact that they are slowly oxidized on exposure to air, However the reactivity of these metals towards oxygen increases on moving down the group.
(iii) The oxides of these metals are very stable due to high lattice energy.
(iv) The oxides of the metal (except BeO and MgO) dissolve in water to form basic hydroxides and evolve a large amount of heat. BeO and MgO possess high lattice energy and thus insoluble in water.
(v) BeO dissolves both in acid and alkalies to give salts i.e. BeO possesses amphoteric nature.
\[BeO+2NaOH\to \underset{\text{Sod}\text{. beryllate}}{\mathop{N{{a}_{2}}Be{{O}_{2}}+{{H}_{2}}O}}\,\,\,;\,\,BeO+2HCl\to \underset{\text{Beryllium chloride}}{\mathop{BeC{{l}_{2}}+{{H}_{2}}O}}\,\]
(vi)The basic nature of oxides of alkaline earth metals increases from Be to Ra as the electropositive Character increases from Be to Ra.
(vii)The tendency of these metal to react with water increases with increase in electropositive character i.e. Be to Ra.
(viii) Reaction of Be with water is not certain, magnesium reacts only with hot water, while other metals react with cold water but slowly and less energetically than alkali metals.
(ix) The inertness of Be and Mg towards water is due to the formation of protective, thin layer of hydroxide on the surface of the metals.
(x) The basic nature of hydroxides increase from Be to Ra. It is because of increase in ionic radius down the group which results in a decrease in strength of M –O bond in M –(OH)2 to show more dissociation of hydroxides and greater basic character.
(xi) The solubility of hydroxides of alkaline earth metals is relatively less than their corresponding alkali metal hydroxides Furthermore, the solubility of hydroxides of alkaline earth metals increases from Be to Ba. Be (OH)2 and Mg (OH)2 are almost insoluble, Ca (OH)2 (often called lime water) is sparingly soluble whereas Sr(OH)2 and Ba (OH)2 (often called baryta water) are more soluble.
The trend of the solubility of these hydroxides depends on the values of lattice energy and hydration energy of these hydroxides. The magnitude of hydration energy remains almost same whereas lattice energy decreases appreciably down the group leading to more –Ve values for \[\Delta {{H}_{\text{solution}}}\] down the group.
\[\Delta {{H}_{\text{solution}}}=\Delta {{H}_{\text{lattice energy}}}+\Delta {{H}_{\text{hydration energy}}}\]
More negative is \[\Delta {{H}_{\text{solution}}}\] more is solubility of compounds.
(xii) The basic character of oxides and hydroxides of alkaline earth metals is lesser than their corresponding alkali metal oxides and hydroxides.
(xiii) Aqueous solution of lime water [Ca(OH)2] or baryta water [Ba(OH)]2 are used to qualitative identification and quantative estimation of carbon dioxide, as both of them gives white precipitate with CO2 due to formation of insoluble CaCO3 or BaCO3
\[Ca{{(OH)}_{2}}+C{{O}_{2}}\to \underset{\text{(white ppt)}}{\mathop{CaC{{O}_{3}}}}\,+{{H}_{2}}O\,\,;\,\,Ba{{(OH)}_{2}}+C{{O}_{2}}\to \underset{\text{(white ppt)}}{\mathop{BaC{{O}_{3}}}}\,+{{H}_{2}}O\]
SO2 also give white ppt of CaSO3 and BaSO3 on passing through lime water or baryta water. However on passing CO2 in excess, the white turbidity of insoluble carbonates dissolve to give a clear solution again due to the formation of soluble bicarbonates,
CaCO3 \[\to \] H2O + CO2 \[\to \] Ca(HCO3)2
(2) Hydrides
(i) Except Be, all alkaline earth metals form hydrides (MH2) on heating directly with H2 . M+ H2 \[\to \] MH2.
(ii) BeH2 is prepared by the action of LiAlH4 On BeCl2
2BeCl2 + LiAlH4 \[\to \] 2BeH2 + LiCl + AlCl3.
(iii) BeH2 and MgH2 are covalent while other hydrides are ionic.
(iv) The ionic hydrides of Ca, Sr, Ba liberate H2 at anode and metal at cathode.
\[Ca{{H}_{2}}C{{a}^{2+}}+2{{H}^{-}}\]
Anode : 2H– \[\to \] H2 + 2e– Cathode : Ca2+ + 2e– \[\to \] Ca
(v) The stability of hydrides decreases from Be to Ba.
(vi) The hydrides having higher reactivity for water, dissolves readily and produce hydrogen gas.
CaH2(s) + 2H2O \[\to \] Ca(OH) 2 + 2H2
(3) Carbonates and Bicarbonates
(i) All these metal carbonates (MCO3) are insoluble in neutral medium but soluble in acid medium. These are precipitated by the addition of alkali metal or ammonium carbonate solution to the solution of these metals.
(NH4)2 CO3 + CaCl2 \[\to \] 2NH4Cl + CaCO3
Na2CO3 + BaCl2 \[\to \]2NaCl + BaCO3
(ii) Alkaline earth metal carbonates are obtained as white precipitates when calculated amount of carbon dioxide is passed through the solution of the alkaline metal hydroxides.
M(OH)2 (aq) + CO2 (g) \[\to \] MCO3(s) + H2O(l)
and sodium or ammonium carbonate is added to the solution of the alkaline earth metal salt such as CaCl2.
CaCl2 (aq) + Na2CO3 (aq) \[\to \] CaCO3(s) +2 NaCl(aq)
(iii) Solubility of carbonates of these metals also decreases downward in the group due to the decrease of hydration energy as the lattice energy remains almost unchanged as in case of sulphates.
(vi) The carbonates of these metals decompose on heating to give the oxides, the temperature of decomposition increasing from Be to Ba. Beryllium carbonate is unstable.
\[MC{{O}_{3}}\xrightarrow{Heat}MO\,+C{{O}_{2}}\]
(4) Halides
(i) The alkaline earth metals combine directly with halogens at appropriate temperatures forming halides, MX2. These halides can also be prepared by the action of halogen acids (HX) on metals, metal oxides, hydroxides and carbonates.
M + 2HX \[\to \] MX2 + H2 ; MO + 2HX \[\to \] MX2 + H2O
M(OH)2 + 2HX \[\to \] MX2 +2H2O
MCO3 + 2HX \[\to \] MX2 + CO2 + H2O
Beryllium chloride is however, conveniently obtained from oxide
\[BeO+C+C{{l}_{2}}\xrightarrow{870-1070K}BeC{{l}_{2}}+CO\]
(ii) BeCl2 is essentially covalent, the chlorides MgCl2, CaCl2 , SrCl2 and BaCl2 are ionic; the ionic character increases as the size of the metal ion increases. The evidence is provided by the following facts,
(a) Beryllium chloride is relatively low melting and volatile whereas BaCl2 has high melting and stable.
(b) Beryllium chloride is soluble in organic solvents.
(iii) The halides of the members of this group are soluble in water and produce neutral solutions from which the hydrates such : MgCl2 6H2O, CaCl2.6H2O. BaCl2 2H2O can be crystallised. The tendency to form hydrated halides decreases with increasing size of the metal ions.
(iv) BeCl2 is readily hydrolysed with water to form acid solution, BeCl2 + 2H2O \[\to \] Be (OH)2 + 2HCl.
(v) The fluorides are relatively less soluble than the chlorides due to high lattice energies. Except BeCl2 and MgCl2 the chlorides of alkaline earth metals impart characteristic colours to flame.
CaCl2 SrCl2 BaCl2
Brick red colour Crimson colour Grassy green colour
Structure of BeCl2 : In the solid phase polymeric chain structure with three centre two electron bonding with Be-Cl-Be bridged structure is shown below,
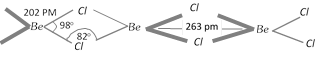
In the vapour phase it tends to form a chloro-bridged dimer which dissociates into the linear triatomic monomer at high temperature at nearly 1200 K.
(5) Solubility in liquid ammonia : Like alkali metals, alkaline earth metals also dissolve in liquid ammonia to form coloured solutions When such a solution is evaporated, hexammoniate, M(NH3)6 is formed.
(6) Nitrides
(i) All the alkaline earth metals direct combine with N2 give nitrides, M3N2.
(ii) The ease of formation of nitrides however decreases from Be to Ba.
(iii) These nitrides are hydrolysed water to liberate
NH3, M3N2 + 6H2O \[\to \] 3M(OH)2 + 2NH3
(7) Sulphates
(i) All these form sulphate of the type M SO4 by the action of H2 SO4 on metals, their oxides, carbonates or hydroxides.
M + H2SO4 \[\to \] MSO4 + H2 ; MO+H2SO4 \[\to \] MSO4 + H2O
MCO3+ H2SO4 \[\to \] MSO4 + H2O + CO2
M(OH)2 + H2SO4 \[\to \] MSO4 + 2H2O
(ii) The solubility of sulphates in water decreases on moving down the group BeSO4 and MgSO4 are fairly soluble in water while BaSO4 is completely insoluble. This is due to increases in lattice energy of sulphates down the group which predominates over hydration energy.
(iii) Sulphate are quite stable to heat however reduced to sulphide on heating with carbon.
MSO4 + 2C \[\to \] MS + 2CO2
(8) Action with carbon : Alkaline metals (except Be, Mg) when heated with carbon form carbides of the type MC2 These carbides are also called acetylides as on hydrolysis they evolve acetylene.
MC2 + 2H2O \[\to \] M(OH) 2 + C2H2
(9) Action with sulphur and phosphorus : Alkaline earth metals directly combine with sulphur and phosphorus when heated to form sulphides of the type MS and phosphides of the type M3P2 respectively.
M + S \[\to \] MS ; 3M + 2P \[\to \] M3P2
Sulphides on hydrolysis liberate H2S while phosphides on hydrolysis evolve phosphine.
MS + dil. acid \[\to \] H2S ; M3P2 + dil. acid \[\to \] PH3
Sulphides are phosphorescent and are decomposed by water
2MS + 2H2O \[\to \] M(OH) 2 + M(HS)2
(10) Nitrates : Nitrates of these metals are soluble in water On heating they decompose into their corresponding oxides with evolution of a mixture of nitrogen dioxide and oxygen.
\[M{{(N{{O}_{3}})}_{2}}\to MO+2N{{O}_{2}}+\left( \frac{1}{2} \right){{O}_{2}}\]
(11) Formation of complexes
(i) Tendency to show complex ion formation depends upon smaller size, high nuclear charge and vacant orbitals to accept electron. Since alkaline metals too do not possess these characteristics and thus are unable to form complex ion.
(ii) However, Be2+ on account of smaller size forms many complex such as (BeF3)1-, (BeF4)2-.
Anomalous behaviour of Beryllium
Beryllium differs from rest of the alkaline earth metals on account of its small atomic size, high electronegativity Be2+ exerts high polarizing effect on anions and thus produces covalent nature in its compounds. Following are some noteworthy difference of Be from other alkaline earth metals,
(1) Be is lightest alkaline earth metal.
(2) Be possesses higher m.pt. and b.pt than other group members.
(3) BeO is amphoteric in nature whereas oxides of other group members are strong base.
(4) It is not easily effected by dry air and does not decompose water at ordinary temperature.
(5) BeSO4 is soluble in water.
(6) Be and Mg carbonates are not precipitated by in presence of NH4Cl.
(7) Be and Mg salts do not impart colour to flame.
(8) Be does not form peroxide like other alkaline earth metals.
(9) It does not evolve hydrogen so readily from acids as other alkaline earth metals do so.
(10) It has strong tendency to form complex compounds.
(11) Be3N2 is volatile whereas nitrides of other alkaline earth metals are non-volatile.
(12) It’s salts can never have more than four molecules of water of crystallization as it has only four available orbitals in its valence shell.
(13) Berylium carbide reacts water to give methane whereas magnesium carbide and calcium carbide give propyne and acetylene respectively.
Be2C + 4H2O \[\to \] 2Be(OH)2 + CH4
Mg2C3 + 4H2O \[\to \] 2Mg(OH)2 + C3H6
CaC2 + 2H2O \[\to \] Ca(OH)2 + C2H4
Diagonal relationship of Be with Al
Due to its small size Be differs from other earth alkaline earth metals but resembles in many of its properties with Al on account of diagonal relationship.
(1) Be2+ and Al3+ have almost same and smaller size and thus favour for covalent bonding.
(2) Both these form covalent compounds having low m. pt and soluble in organic solvent.
(3) Both have same value of electronegativity (i.e. 1.5).
(4) The standard O.P of these elements are quite close to each other ; Be2+ = 1.69 volts and Al3+ = 1.70 volts.
(5) Both become passive on treating with conc. HNO3 in cold.
(6) Both form many stable complexes e.g. (BeF3)–, (AlH4)–.
(7) Like BeO, Al2O3 is amphoteric in nature. Also both are high melting point solids.
Al2O3 + 2NaOH \[\to \] 2NaAlO2 + H2O
Al2O3 + 6HCl \[\to \] 2AlCl3 + 3H2O
(8) Be and Al both react with NaOH to liberate H2 forming beryllates and alluminates.
Be + 2NaOH \[\to \] Na2BeO2 + H2
2Al + 6NaOH \[\to \] 2Na3AlO3 + 3H2
(9) Be2C and Al4C3 both give CH4 on treating with water.
Be2C + 2H2O \[\to \] CH4 + 2BeO
Al4C3 + 6H2O \[\to \] 3CH4 + 2Al2O3
(10) Both occur together in nature in beryl ore, 3BeO. Al2O3. 6SiO2.
(11) Unlike other alkaline earths but like aluminium, beryllium is not easily attacked by air (Also Mg is not attacked by air)
(12) Both Be and Al react very slowly with dil. HCl to liberate H2.
(13) Both Be and Al form polymeric covalent hydrides while hydrides of other alkaline earth are ionic.
(14) Both BeCl2 and AlCl3 are prepared is similar way.
BeO+ C+ Cl2 \[\to \] BeCl2 + CO
Al2O3 + 3C +3Cl2 \[\to \] 2AlCl3 + 3CO
(15) Both BeCl2 and AlCl3 are soluble in organic solvents and act as catalyst in Friedel –Crafts reaction.
(16) Both Be (OH)2 and Al (OH)3 are amphoteric whereas hydroxides of other alkaline earths are strong alkali.
(17) The salts of Be and Al are extensively hydrated.
(18) BeCl2 and AlCl3 both have a bridged polymeric structure.
(19) Be and Al both form fluoro complex ions [BeF4]2– and [AlF6]3– in solution state whereas other members of 2nd group do not form such complexes.
Magnesium and its compounds
(1) Ores of magnesium : Magnesite \[(MgC{{O}_{3}}),\] Dolomite \[(MgC{{O}_{3}}.CaC{{O}_{3}})\], Epsomite (epsom salt) \[(MgS{{O}_{4}}.7{{H}_{2}}O)\] Carnallite \[(MgC{{l}_{2}}.\,KCl.\,6{{H}_{2}}O)\] Asbestos \[(CaM{{g}_{3}}{{(Si{{O}_{3}})}_{4}}),\] Talc \[(M{{g}_{2}}\]\[{{(S{{i}_{2}}{{O}_{5}})}_{2}}.\,Mg{{(OH)}_{2}})\].
(2) Extraction of magnesium : It is prepared by the electrolysis of fused magnesium chloride which in turn is obtained from carnallite and magnesite.
Carnallite \[(MgC{{l}_{2}}.KCl.6{{H}_{2}}O)\] can’t be directly converted into anhydrous \[MgC{{l}_{2}}\] by heating because all the water of crystallisation cannot be removed by heating. Moreover, strong heating may change it to \[MgO\].
\[MgC{{l}_{2}}+2{{H}_{2}}O\xrightarrow{\Delta }MgO\,+2HCl+{{H}_{2}}O\]
In Dow’s process, magnesium chloride is obtained from sea water as \[MgC{{l}_{2}}.6{{H}_{2}}O\]. It is rendered anhydrous by heating it in a current of dry \[HCl\] gas. The anhydrous magnesium chloride is fused with \[NaCl\] (to provide conductivity to the electrolyte and to lower the fusing temperature of anhydrous \[MgC{{l}_{2}}\]) and then electrolysed at \[{{700}^{o}}C\].
(3) Compounds of magnesium
(i) Magnesia (MgO) : It is used as magnesia cement. It is a mixture of \[MgO\] and \[MgC{{l}_{2}}.\] It is also called Sorel's cement.
(ii) Magnesium hydroxide : It aqueous suspension is used in Medicine as an antacid. Its medicinal name is milk of magnesia.
(iii) Magnesium sulphate or Epsom salt \[(MgS{{O}_{4}}.\,7{{H}_{2}}O)\]: It is isomorphous with \[ZnS{{O}_{4}}.\,7{{H}_{2}}O.\] It is used as a purgative in medicine, as a mordant in dyeing and as a stimulant to increase the secretion of bile.
(iv) Magnesium chloride \[(MgC{{l}_{2}}.6{{H}_{2}}O)\]: It is a deliquescent solid. Hydrated salt on heating in air undergoes partial hydrolysis.
\[MgC{{l}_{2}}.\,6{{H}_{2}}O\xrightarrow{\text{Heat}}Mg(OH)Cl+HCl+5{{H}_{2}}O\].
Calcium and its compounds
(1) Ores of calcium : Lime stone or marble or chalk \[(CaC{{O}_{3}}),\]Gypsum\[(CaS{{O}_{4}}.\,2{{H}_{2}}O),\,\] Dolomite \[(CaC{{O}_{3}}.\,MgC{{O}_{3}}),\] Fluorspar \[(Ca{{F}_{2}}),\] phosphorite \[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}\]. Calcium phosphate is a constituent of bones and teeth.
(2) Manufacture : It is manufactured by the electrolysis of a molten mixture of calcium chloride containing some calcium fluoride. Calcium chloride is obtained as a by product of the solvay process.
(3) Compounds of calcium
(i) Calcium oxide or Quick lime or Burnt lime (CaO) : It's aqueous suspension is known as slaked lime.
\[CaO+{{H}_{2}}O\]\[\xrightarrow[{}]{\text{hissing sound}}\]\[\underset{\begin{smallmatrix} \text{slaked} \\ \text{ lime} \end{smallmatrix}}{\mathop{Ca{{(OH)}_{2}}}}\,+\text{Heat},\]
(iv) Magnesium chloride : It is a deliquescent solid. Hydrated salt on heating in air undergoes partial hydrolysis.
Calcium and its compounds
(1) Ores of calcium : Lime stone or marble or chalk Gypsum Dolomite Fluorspar phosphorite . Calcium phosphate is a constituent of bones and teeth.
(2) Manufacture : It is manufactured by the electrolysis of a molten mixture of calcium chloride containing some calcium fluoride. Calcium chloride is obtained as a by product of the solvay process.
(3) Compounds of calcium
(i) Calcium oxide or Quick lime or Burnt lime (CaO) : It's aqueous suspension is known as slaked lime.
\[CaO+{{H}_{2}}O\xrightarrow{\text{hissing sound}}\underset{\begin{smallmatrix}\text{slaked } \\ \,\,\text{lime} \end{smallmatrix}}{\mathop{Ca{{(OH)}_{2}}}}\,+\]
When exposed to oxy-hydrogen flame, it starts emitting light called lime light.
\[CaO\] is used as basic flux, for removing hardness of water, as a drying agent (for \[N{{H}_{3}}\]gas) for preparing mortar (CaO+ sand +water).
Mortar : Mortar used in making buildings is a mixture of lime (CaO) and sand in the ratio 1 : 3 with enough water to make a thick paste. When the mortar is placed between bricks, it slowly absorbs CO2 from the air and the slaked lime revers to CaCO3.
\[Ca{{(OH)}_{2}}(s)+C{{O}_{2}}(g)\to CaC{{O}_{3}}(s)+{{H}_{2}}O(l)\]
Although the sand in the mortar is chemically inert, the grains are bound together by the particles of calcium carbonate and a hard material results.
(ii) Calcium chloride \[(CaC{{l}_{2}}.6{{H}_{2}}O):\] Fused \[CaC{{l}_{2}}\] is a good dessicant (drying agent). It can't be used to dry alcohol or ammonia as it forms additional products with them.
(iii) Calcium carbonate (CaCO3) :
\[Ca{{(OH)}_{2}}+C{{O}_{2}}\to CaC{{O}_{3}}+{{H}_{2}}O\].
It is insoluble in water but dissolves in the presence of \[C{{O}_{2}}\] due to the formation of calcium bicarbonate.
\[CaC{{O}_{3}}+{{H}_{2}}O+C{{O}_{2}}\to Ca{{(HC{{O}_{3}})}_{2}}\]
It is a constituent of protective shells of marine animals.
(iv) Gypsum \[(CaS{{O}_{4}}.\,2{{H}_{2}}O):\] On partially dehydrates to produce plaster of paris.
\[\underset{\text{Gypsum}}{\mathop{CaS{{O}_{4}}}}\,.\,2{{H}_{2}}O\xrightarrow{{{120}^{\,o}}C}\underset{\begin{smallmatrix} \text{Plaster of } \\\text{paris}\end{smallmatrix}}{\mathop{CaS{{O}_{4}}}}\,.\frac{1}{2}{{H}_{2}}O+1\frac{1}{2}{{H}_{2}}O\]
Plaster of paris :
\[\underset{\text{Plaster of paris}}{\mathop{CaS{{O}_{4}}.\frac{1}{2}{{H}_{2}}O}}\,\underset{\text{Setting}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{orthorhombic}}{\mathop{CaS{{O}_{4}}.\,2{{H}_{2}}O}}\,\xrightarrow{\text{Hardening }}\underset{\text{Monoclinic (gypsum)}}{\mathop{CaS{{O}_{4}}.2{{H}_{2}}O}}\,\]
\[\underset{\text{Gypsum}}{\mathop{CaS{{O}_{4}}.2{{H}_{2}}O}}\,\]\[\xrightarrow{{{200}^{o}}C}\underset{\text{dead burnt plaster}}{\mathop{CaS{{O}_{4}}\text{(anhydrous)}}}\,\]
Gypsum when heated to about \[{{200}^{o}}C\] is converted into anhydrous calcium sulphate. The anhydrous form (anhydrite) is known as dead burnt plaster because it does not set like plaster of paris when moistened with water.
(v) Calcium Hydroxide \[Ca{{(OH)}_{2}}\] (slaked lime)
\[CaO+{{H}_{2}}O\xrightarrow{\text{hissing sound}}\underset{\begin{smallmatrix}\text{slaked } \\ \,\,\text{lime} \end{smallmatrix}}{\mathop{Ca{{(OH)}_{2}}}}\,+\]
(vi) Cement : (a) It is essentially a mixture of lime stone and clay. It is also called Portland cement because in presence of water it sets to a hard stone-like mass resembling with the famous Portland rock, a famous building stone of England. The approximate composition of cement is
Calcium oxide \[(CaO)\] 50 – 60 %
Silica \[(Si{{O}_{2}})\] 20 – 25%
Alumina \[(A{{l}_{2}}{{O}_{3}})\] 5 – 10%
Magnesia \[(MgO)\] 1 – 3%
Ferric oxide \[(F{{e}_{2}}{{O}_{3}})\] 1 – 3%
The above compounds are provided by the two raw materials, namely lime stone (which provides \[CaO\]) and clay which provides \[Si{{O}_{2}},\,A{{l}_{2}}{{O}_{3}}\] and \[F{{e}_{2}}{{O}_{3}}\]. In cement, almost entire amount of lime is present in the combined state as calcium silicates \[(2CaO.Si{{O}_{2}}\] and \[3CaO.Si{{O}_{2}})\] and calcium aluminates \[(3CaO.A{{l}_{2}}{{O}_{3}}\] and \[4CaO.A{{l}_{2}}{{O}_{3}})\].
(b) Cement containing excess amount of lime cracks during setting; while cement containing less amount of lime is weak in strength.
(c) Cement with excess of silica is slow-setting and that having an excess of alumina is quick-setting.
(d) Cement containing no iron oxide is white but hard to burn.
Cement is manufactured by two processes, viz, wet and dry. A small amount (2–3%) of gypsum is added to slow down the setting of the cement so that it gets sufficiently hardened. Setting of cement is an exothermic process and involves hydration of calcium aluminates and calcium silicates.
You need to login to perform this action.
You will be redirected in
3 sec
