Charles Law
Category : JEE Main & Advanced
(1) French chemist, Jacques Charles first studied variation of volume with temperature, in 1787.
(2) It states that, “The volume of a given mass of a gas is directly proportional to the absolute temperature \[(={{\,}^{o}}C+273)\] at constant pressure”.
Thus, \[V\propto T\] at constant pressure and mass
or \[V=KT=K(t({{\,}^{o}}C)+273.15)\] , (where k is constant),
\[K=\frac{V}{T}\] or \[\frac{{{V}_{1}}}{{{T}_{1}}}=\frac{{{V}_{2}}}{{{T}_{2}}}=K\] (For two or more gases)
(3) If \[t={{0}^{o}}C\], then \[V={{V}_{0}}\]
hence, \[{{V}_{0}}=K\times 273.15\]
\ \[K=\frac{{{V}_{0}}}{273.15}\]
\[V=\frac{{{V}_{0}}}{273.15}[t+273.15]={{V}_{0}}\left[ 1+\frac{t}{273.15} \right]={{V}_{0}}[1+{{\alpha }_{v}}t]\]
where \[{{\alpha }_{v}}\] is the volume coefficient,
\[{{\alpha }_{v}}=\frac{V-{{V}_{0}}}{t{{V}_{0}}}=\frac{1}{273.15}=3.661\times {{10}^{-3}}{{\,}^{o}}{{C}^{-1}}\]
Thus, for every \[{{1}^{o}}\] change in temperature, the volume of a gas changes by \[\frac{1}{273.15}\left( \approx \frac{1}{273} \right)\] of the volume at \[{{0}^{o}}C\].
(4) Graphical representation of Charle's law : Graph between V and T at constant pressure is called isobar or isoplestics and is always a straight line. A plot of V versus \[t({{\,}^{o}}C)\] at constant pressure is a straight line cutting the temperature axis at \[-{{273.15}^{o}}C\]. It is the lowest possible temperature.
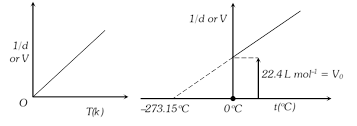
(5) At constant mass and pressure density of a gas is inversely proportional to it absolute temperature.
Thus, \[d\propto \frac{1}{T}\propto \frac{1}{V}\] \[\left[ \because V=\frac{\text{mass}}{\text{d}} \right]\]
or \[\frac{{{d}_{1}}}{{{d}_{2}}}=\frac{{{T}_{2}}}{{{T}_{1}}}=\frac{{{V}_{2}}}{{{V}_{1}}}=......=K\]
(6) Use of hot air balloons in sports and meteorological observations is an application of Charle's law.
You need to login to perform this action.
You will be redirected in
3 sec
