Gas Laws
Category : JEE Main & Advanced
(1) Boyle's law : For a given mass of an ideal gas at constant temperature, the volume of a gas is inversely proportional to its pressure.
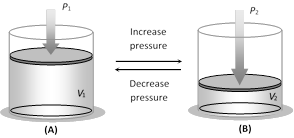
i.e. \[V\propto \frac{1}{P}\] or PV = constant \[\Rightarrow \] \[{{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}\]
(i) \[PV=P\,\left( \frac{m}{\rho } \right)=\] constant \[\Rightarrow \] \[\frac{P}{\rho }=\text{constant}\] or \[\frac{{{P}_{1}}}{{{\rho }_{1}}}=\frac{{{P}_{2}}}{{{\rho }_{2}}}\]
(As volume \[=\frac{m}{\rho (\text{Density of the gas)}}\]and m = constant)
(ii) \[PV=P\left( \frac{N}{n} \right)=\text{constant}\] \[\Rightarrow \] \[\frac{P}{n}=\text{constant}\] or \[\frac{{{P}_{1}}}{{{n}_{1}}}=\frac{{{P}_{2}}}{{{n}_{2}}}\]
(iii) As number of molecules per unit volume \[n=\frac{N}{V}\]
\[\Rightarrow \] \[V=\frac{N}{n}\] also N = constant
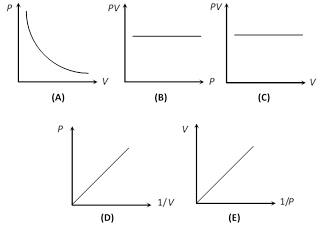
(iv) Graphical representation : If m and T are constant
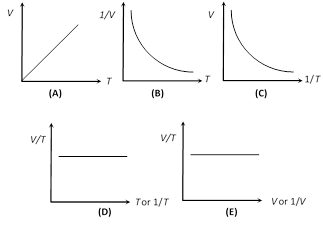
(2) Charle's law : If the pressure remaining constant, the volume of the given mass of a gas is directly proportional to its absolute temperature.
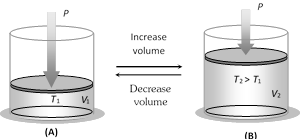
i.e., \[V\propto T\] \[\Rightarrow \] \[\frac{V}{T}=\text{constant}\]\[\Rightarrow \]\[\frac{{{V}_{1}}}{{{T}_{1}}}=\frac{{{V}_{2}}}{{{T}_{2}}}\]
(i) \[\frac{V}{T}=\]\[\frac{m}{\rho T}=\text{constant}\] (As volume \[V=\frac{m}{\rho }\])
or \[\rho T=\text{constant}\]\[\Rightarrow \]\[{{\rho }_{1}}{{T}_{1}}={{\rho }_{2}}{{T}_{2}}\]
(ii) If the pressure remains constant, the volume of the given mass of a gas increases or decreases by \[\frac{1}{273.15}\] of its volume at \[{{0}^{o}}C\] for each \[{{1}^{o}}C\] rise or fall in temperature.
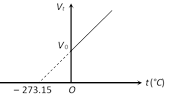
\[{{V}_{t}}={{V}_{0}}\left( 1+\frac{1}{273.15}t \right)\].
This is Charle?s law for centigrade scale. (v) Graphical representation: If m and P are constant
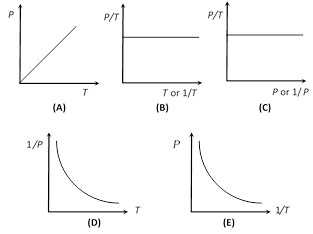
(3) Gay-Lussac's law or pressure law : The volume remaining constant, the pressure of a given mass of a gas is directly proportional to its absolute temperature.
\[P\propto T\] or \[\frac{P}{T}=\text{constant}\] \[\Rightarrow \] \[\frac{{{P}_{1}}}{{{T}_{1}}}=\frac{{{P}_{2}}}{{{T}_{2}}}\]
(i) The volume remaining constant, the pressure of a given mass of a gas increases or decreases by \[\frac{1}{273.15}\] of its pressure at \[{{0}^{o}}C\] for each \[{{1}^{o}}C\] rise or fall in temperature.
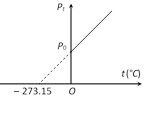
\[{{P}_{t}}={{P}_{0}}\left[ 1+\frac{1}{273.15}t \right]\]
This is pressure law for centigrade scale.
(ii) Graphical representation : If m and V are constants
(4) Avogadro's law : Equal volume of all the gases under similar conditions of temperature and pressure contain equal number of molecules i.e. \[{{N}_{1}}={{N}_{2}}\].
(5) Grahm's law of diffusion : When two gases at the same pressure and temperature are allowed to diffuse into each other, the rate of diffusion of each gas is inversely proportional to the square root of the density of the gas i.e. \[r\propto \frac{1}{\sqrt{\rho }}\] \[\propto \] \[\frac{1}{\sqrt{M}}\]
(M is the molecular weight of the gas) \[\Rightarrow \] \[\frac{{{r}_{1}}}{{{r}_{2}}}=\sqrt{\frac{{{\rho }_{2}}}{{{\rho }_{1}}}}\]\[=\sqrt{\frac{{{M}_{2}}}{{{M}_{1}}}}\]
If V is the volume of gas diffused in t sec then
\[r=\frac{V}{t}\]\[\Rightarrow \]\[\frac{{{r}_{1}}}{{{r}_{2}}}=\frac{{{V}_{1}}}{{{V}_{2}}}\times \frac{{{t}_{2}}}{{{t}_{1}}}\]
(6) Dalton?s law of partial pressure : The total pressure exerted by a mixture of non-reacting gases occupying a vessel is equal to the sum of the individual pressures which each gases exert if it alone occupied the same volume at a given temperature.
For n gases \[P={{P}_{1}}+{{P}_{2}}+{{P}_{3}}+.....{{P}_{n}}\]
where P = Pressure exerted by mixture and \[{{P}_{1}},\,{{P}_{2}},\,{{P}_{3}},\,......{{P}_{n}}=\]Partial pressure of component gases.
You need to login to perform this action.
You will be redirected in
3 sec
