Pressure of an Ideal Gas
Category : JEE Main & Advanced
Consider an ideal gas (consisting of N molecules each of mass m) enclosed in a cubical box of side L.
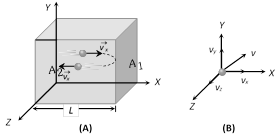
(1) Instantaneous velocity : Any molecule of gas moves with velocity \[\vec{v}\] in any direction
Where \[\vec{v}={{v}_{x}}\hat{i}+{{v}_{y}}\hat{j}+{{v}_{z}}\hat{k}\] \[\Rightarrow \] \[v=\sqrt{v_{x}^{2}+v_{y}^{2}+v_{z}^{2}}\]. Due to random motion of molecule \[{{v}_{x}}={{v}_{y}}={{v}_{z}}\] \[\Rightarrow \] \[{{v}^{2}}=3v_{x}^{2}=3v_{y}^{2}=3v_{z}^{2}\]
(2) Time during collision : Time between two successive collision with the wall \[{{A}_{1}}\].
\[\Delta t=\frac{\text{Distance travelled by molecule between two successive collision}}{\text{Velocity of molecule}}\]
\[=\frac{2L}{{{v}_{x}}}\]
(3) Collision frequency (n) : It means the number of collision per second. Hence \[n=\frac{1}{\Delta t}=\frac{{{v}_{x}}}{2L}\]
(4) Change in momentum : This molecule collides with the shaded wall \[({{A}_{1}})\] with velocity \[{{v}_{x}}\] and rebounds with velocity \[-{{v}_{x}}\].
The change in momentum of the molecule
\[\Delta p=(-m{{v}_{x}})-(m{{v}_{x}})=-2m{{v}_{x}}\]
As the momentum remains conserved in a collision, the change in momentum of the wall \[{{A}_{1}}\] is \[\Delta p=2m{{v}_{x}}\]
After rebound this molecule travel toward opposite wall \[{{A}_{2}}\] with velocity \[-{{v}_{x}}\], collide to it and again rebound with velocity \[{{v}_{x}}\] towards wall \[{{A}_{1}}\].
(5) Force on wall : Force exerted by a single molecule on shaded wall is equal to rate at which the momentum is transferred to the wall by this molecule.
i.e. \[{{F}_{\text{Single molecule}}}=\frac{\Delta p}{\Delta t}=\frac{2m{{v}_{x}}}{(2L/{{v}_{x}})}=\frac{mv_{x}^{2}}{L}\]
The total force on the wall \[{{A}_{1}}\] due to all the molecules
\[{{F}_{x}}=\frac{m}{L}\sum{v_{x}^{2}}\]\[=\frac{m}{M}(v_{{{x}_{1}}}^{2}+v_{{{x}_{2}}}^{2}+v_{{{x}_{3}}}^{2}+...)=\frac{mN}{L}\overline{v_{x}^{2}}\]
\[\overline{v_{x}^{2}}=\]mean square of \[x\] component of the velocity.
(6) Pressure : Now pressure is defined as force per unit area, hence pressure on shaded wall \[{{P}_{x}}=\frac{{{F}_{x}}}{A}=\frac{mN}{AL}\overline{v_{x}^{2}}=\frac{mN}{V}\overline{v_{x}^{2}}\]
For any molecule, the mean square velocity \[\overline{{{v}^{2}}}=\overline{v_{x}^{2}}+\overline{v_{y}^{2}}+\overline{v_{z}^{2}}\]; by symmetry
\[\overline{v_{x}^{2}}=\overline{v_{y}^{2}}=\overline{v_{z}^{2}}\]
\[\Rightarrow\]\ \overline{v_{x}^{2}}=\overline{v_{y}^{2}}=\overline{v_{z}^{2}}=\frac{\overline{{{v}^{2}}}}{3}\]
Total pressure inside the container
\[P=\frac{1}{3}\frac{mN}{V}\overline{{{v}^{2}}}=\frac{1}{3}\frac{m\,N}{V}v_{rms}^{2}\] (where \[{{v}_{rms}}=\sqrt{\overline{{{v}^{2}}}}\])
(7) Relation between pressure and kinetic energy : As we know \[P=\frac{1}{3}\frac{m\,N}{V}v_{rms}^{2}\]\[=\frac{1}{3}\frac{M}{V}v_{rms}^{2}\]\[\Rightarrow \]\[P=\frac{1}{3}\rho \,v_{rms}^{2}\] ... (i)
[As M = mN = Total mass of the gas and \[\rho =\frac{M}{V}\]]
\[\therefore \] K.E. per unit volume \[E=\frac{1}{2}\left( \frac{M}{V} \right)\,v_{rms}^{2}=\frac{1}{2}\rho \,v_{rms}^{2}\] ...(ii)
From (i) and (ii), we get \[P=\frac{2}{3}E\]
i.e. the pressure exerted by an ideal gas is numerically equal to the two third of the mean kinetic energy of translation per unit volume of the gas.
(8) Effect of mass, volume and temperature on pressure : \[P=\frac{1}{3}\frac{m\,N}{V}v_{rms}^{2}\] or \[P\propto \frac{(m\,N)T}{V}\] [As \[v_{rms}^{2}\propto T\]]
(i) If volume and temperature of a gas are constant \[P\propto mN\] i.e. Pressure µ (Mass of gas). i.e. if mass of gas is increased, number of molecules and hence number of collision per second increases i.e. pressure will increase.
(ii) If mass and temperature of a gas are constant. \[P\propto (1/V)\], i.e., if volume decreases, number of collisions per second will increase due to lesser effective distance between the walls resulting in greater pressure.
(iii) If mass and volume of gas are constant, \[P\propto {{({{v}_{rms}})}^{2}}\propto T\]
i.e., if temperature increases, the mean square speed of gas molecules will increase and as gas molecules are moving faster, they will collide with the walls more often with greater momentum resulting in greater pressure.
You need to login to perform this action.
You will be redirected in
3 sec
