Mass Defect and Binding Energy
Category : JEE Main & Advanced
(1) Mass defect \[(\Delta m)\]: It is found that the mass of a nucleus is always less than the sum of masses of it's constituent nucleons in free state. This difference in masses is called mass defect. Hence mass defect
\[\Delta m=\] Sum of masses of nucleons - Mass of nucleus
\[=\left\{ Z{{m}_{p}}+(A-Z){{m}_{n}} \right\}-M=\left\{ Z{{m}_{p}}+Z{{m}_{e}}+(A-Z){{m}_{z}} \right\}-M'\]
where \[{{m}_{p}}=\] Mass of proton, \[{{m}_{n}}=\]Mass of each neutron, \[{{m}_{e}}=\]Mass of each electron
M = Mass of nucleus, Z = Atomic number, A = Mass number, M¢ = Mass of atom as a whole.
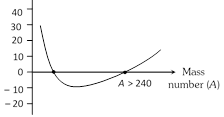
(2) Packing fraction : Mass defect per nucleon is called packing fraction
Packing fraction (f ) \[=\frac{\Delta m}{A}=\frac{M-A}{A}\] where M = Mass of nucleus, A = Mass number
Packing fraction measures the stability of a nucleus. Smaller the value of packing fraction, larger is the stability of the nucleus.
(i) Packing fraction may be of positive, negative or zero value.
(ii) At A = 16, f\[\to \] Zero
(3) Binding energy (B.E.) : The neutrons and protons in a stable nucleus are held together by nuclear forces and energy is needed to pull them infinitely apart (or the same energy is released during the formation of the nucleus). This energy is called the binding energy of the nucleus.
or
The binding energy of a nucleus may be defined as the energy equivalent to the mass defect of the nucleus.
If \[\Delta m\] is mass defect then according to Einstein's mass energy relation
Binding energy \[=\Delta m\cdot {{c}^{2}}=[\{{{m}_{p}}Z+{{m}_{n}}(A-Z)\}-M]\cdot {{c}^{2}}\]
(This binding energy is expressed in joule, because \[\Delta m\]is measured in kg)
If \[\Delta m\] is measured in amu then binding energy \[=\Delta m\,\,amu\]\[=[\{{{m}_{p}}Z+{{m}_{n}}(A-Z)-M]amu=\Delta m\times 931\,\,MeV\]
(4) Binding energy per nucleon : The average energy required to release a nucleon from the nucleus is called binding energy per nucleon.
Binding energy per nucleon
\[=\frac{\text{Total binding energy}}{\begin{matrix} \text{Mass number (}i.e\text{. total number} \\ \text{ of nucleons)} \\ \end{matrix}}\]\[=\frac{\Delta m\times 931}{A}\frac{MeV}{Nucleon}\]
Binding energy per nucleon \[\propto \] Stability of nucleus
You need to login to perform this action.
You will be redirected in
3 sec
