Nuclear Radiations
Category : JEE Main & Advanced
According to Rutherford's experiment when a sample of radioactive substance is put in a lead box and allow the emission of radiation through a small hole only. When the radiation enters into the external electric field, they splits into three parts (\[\alpha -\]rays, \[\beta -\]rays and \[\gamma -\]rays)
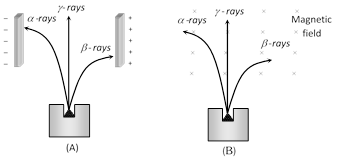
(1) \[\alpha -\]decay : Nearly 90% of the 2500 known nuclides are radioactive ; they are not stable but decay into other nuclides
(i) When unstable nuclides decay into different nuclides, they usually emit alpha \[(\alpha )\] or beta \[(\beta )\] particles.
(ii) Alpha emission occurs principally with nuclei that are too large to be stable. When a nucleus emits an alpha particle, its N and Z values each decrease by two and A decreases by four.
(iii) Alpha decay is possible whenever the mass of the original neutral atom is greater than the sum of the masses of the final neutral atom and the neutral helium- atom.
(2) \[\beta -\]decay : There are different simple type of \[\beta -\]decay \[{{\beta }^{-}}\], \[{{\beta }^{+}}\] and electron capture.
(i) A beta minus particle \[({{\beta }^{-}})\] is an electron. Emission of \[{{\beta }^{-}}\] involves transformation of a neutron into a proton, an electron and a third particle called an antineutrino \[(\bar{\nu })\].
(ii) \[{{\beta }^{-}}\] decay usually occurs with nuclides for which the neutron to proton ratio \[\left( \frac{N}{Z}ratio \right)\] is too large for stability.
(iii) In \[{{\beta }^{-}}\] decay, N decreases by one, Z increases by one and A doesn't change.
(iv) \[{{\beta }^{-}}\] decay can occur whenever the neutral atomic mass of the original atom is larger than that of the final atom.
(v) Nuclides for which N/Z is too small for stability can emit a positron, the electron's antiparticle, which is identical to the electron but with positive charge. The basic process called beta plus \[{{\beta }^{+}}\] decay
\[p\to n+{{\beta }^{+}}+\nu \] (n = neutrino)
(vi) \[{{\beta }^{+}}\] decay can occur whenever the neutral atomic mass of the original atom is at least two electron masses larger than that of the final atom
(vii) The mass of n and \[\bar{\nu }\] is zero. The spin of both is \[\frac{1}{2}\] in units of \[\frac{h}{2\pi }.\] The charge on both is zero. The spin of neutrino is antiparallel to it's momentum while that of antineutrino is parallel to it's momentum.
(viii) There are a few nuclides for which \[{{\beta }^{+}}\] emission is not energetically possible but in which an orbital electron (usually in the k-shell) can combine with a proton in the nucleus to form a neutron and a neutrino. The neutron remains in the nucleus and the neutrino is emitted.
\[p+{{\beta }^{+}}\to n+\nu \]
(3) \[\gamma -\]decay : The energy of internal motion of a nucleus is quantized. A typical nucleus has a set of allowed energy levels, including a ground state (state of lowest energy) and several excited states. Because of the great strength of nuclear interactions, excitation energies of nuclei are typically of the order of the order of 1 MeV, compared with a few eV for atomic energy levels. In ordinary physical and chemical transformations the nucleus always remains in its ground state. When a nucleus is placed in an excited state, either by bombardment with high-energy particles or by a radioactive transformation, it can decay to the ground state by emission of one or more photons called gamma rays or gamma-ray photons, with typical energies of 10 keV to 5 MeV. This process is called gamma \[(\gamma )\] decay.
All the known conservation laws are obeyed in \[\gamma -\]decay.
The intensity of \[\gamma -\]decay after passing through \[x\] thickness of a material is given by \[I={{I}_{0}}{{e}^{-\mu x}}\] (\[\mu =\]absorption co-efficient)
You need to login to perform this action.
You will be redirected in
3 sec
