Radioactive Disintegration
Category : JEE Main & Advanced
(1) Law of radioactive disintegration : According to Rutherford and Soddy law for radioactive decay is as follows.
"At any instant the rate of decay of radioactive atoms is proportional to the number of atoms present at that instant" i.e.
\[-\frac{dN}{dt}\propto N\]\[\Rightarrow \]\[\frac{dN}{dt}=-\lambda N\]. It can be proved that \[N={{N}_{0}}{{e}^{\lambda t}}\]
In terms of mass \[M={{M}_{0}}{{e}^{-\lambda t}}\]
where N = Number of atoms remains undecayed after time t, \[{{N}_{0}}=\] Number of atoms present initially (i.e. at \[t=0\]), M = Mass of radioactive nuclei at time t, \[{{M}_{0}}=\] Mass of radioactive nuclei at time \[t=0,\,\,{{N}_{0}}-N=\] Number of disintegrated nucleus in time t
\[\frac{dN}{dt}\]= rate of decay, \[\lambda =\] Decay constant or disintegration constant or radioactivity constant or Rutherford Soddy's constant or the probability of decay per unit time of a nucleus.
Properties of \[\alpha ,\,\,\beta \] and \[\gamma -\]rays
| Features | \[\alpha -\]particles | \[\beta -\] particles | \[\gamma -\] rays |
| 1. Identity | Helium nucleus or doubly ionised helium atom \[{{(}_{2}}H{{e}^{4}})\] | Fast moving electron \[(-{{\beta }^{0}}\text{ or }{{\beta }^{\text{--}}})\] | Photons (E.M. waves) |
| 2. Charge | \[+2e\] | \[-e\] | Zero |
| 3. Mass \[4\,{{m}_{p}}\](\[{{m}_{p}}=\] mass of proton \[=1.87\times {{10}^{-27}}\] | \[4\,\,{{m}_{p}}\] | \[{{m}_{e}}\] | Massless |
| 4. Speed | \[\approx {{10}^{7}}\,m/s\] | 1% to 99% of speed of light | Speed of light |
| 5. Range of kinetic energy | 4 MeV to 9 MeV | All possible values between a minimum certain value to 1.2 MeV | Between a minimum value to 2.23 MeV |
| 6. Penetration power \[(\gamma ,\,\,\,\beta ,\,\,\alpha )\] | 1 (Stopped by a paper) | 100 (100 times of \[\alpha \]) | 10,000 (100 times of \[\beta \] upto 30 cm of iron (or Pb) sheet |
| 7. Ionisation power \[(\alpha >\beta >\gamma )\] | 10,000 | 100 | 1 |
| 8. Effect of electric or magnetic field | Deflected | Deflected | Not deflected |
| 9. Energy spectrum | Line and discrete | Continuous | Line and discrete |
| 10. Mutual interaction with matter | Produces heat | Produces heat | Produces, photo-electric effect, Compton effect, pair production |
| 11. Equation of decay | \[_{Z}{{X}^{A}}\xrightarrow{\alpha -decay}\] \[_{Z-2}{{Y}^{A-4}}+{{\,}_{2}}H{{e}^{4}}\] \[_{Z}{{X}^{A}}\xrightarrow{{{n}_{\alpha }}}{{\,}_{Z'}}{{Y}^{A'}}\] \[\Rightarrow \] \[{{n}_{\alpha }}=\frac{A-A'\,}{\mathbf{4}}\] | \[_{Z}{{X}^{A}}\to {{\,}_{Z+1}}{{Y}^{A}}+{{\,}_{-1}}{{e}^{0}}+\bar{\nu }\] \[_{Z}{{X}^{A}}\xrightarrow{{{n}_{\beta }}}{{\,}_{Z'}}{{X}^{A}}\]\[\Rightarrow {{n}_{\beta }}=\mathbf{(2}{{n}_{\alpha }}-Z+Z'\mathbf{)}\] | \[_{Z}{{X}^{A}}\to {{\,}_{Z}}{{X}^{a}}+\gamma \] |
(2) Activity : It is defined as the rate of disintegration (or count rate) of the substance (or the number of atoms of any material decaying per second) i.e.
\[A=-\frac{dN}{dt}=\lambda N=\lambda {{N}_{0}}{{e}^{-\lambda t}}={{A}_{0}}{{e}^{-\lambda t}}\]
where A0 = Activity of t = 0, A = Activity after time t
Units of activity (Radioactivity)
It's units are Becqueral (Bq), Curie (Ci) and Rutherford (Rd)
1 Becquerel = 1 disintegration/sec,
1 Rutherford = 106 dis/sec, 1 Curie = 3.7 x 1011 dis/sec
(3) Half life (T1/2) : Time interval in which the mass of a radioactive substance or the number of it's atom reduces to half of it's initial value is called the half life of the substance.
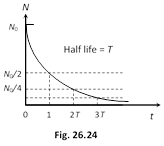
i.e. if \[N=\frac{{{N}_{0}}}{2}\]
then \[t={{T}_{1/2}}\]
Hence from \[N={{N}_{0}}{{e}^{-\lambda t}}\]
\[\frac{{{N}_{0}}}{2}={{N}_{0}}{{e}^{-\lambda \,({{T}_{1/2}})}}\] Þ \[{{T}_{1/2}}=\frac{{{\log }_{e}}2}{\lambda }=\frac{0.693}{\lambda }\]
Fraction of active/decayed atom at different time
| Time (t) | Remaining fraction of active atoms (N/N0) probability of survival | Fraction of atoms decayed (N0 ? N) /N0 probability of decay |
| t = 0 | 1 (100%) | 0 |
| t = T1/2 | \[\frac{1}{2}\] (50%) | \[\frac{1}{2}\] (50%) |
| t = 2(T1/2) | \[\frac{1}{4}\] (25%) | \[\frac{3}{4}\] (75%) |
| t = 3(T1/2) | \[\frac{1}{8}\] (12.5%) | \[\frac{7}{8}\] (87.5%) |
| t = 10 (T1/2) | \[{{\left( \frac{1}{2} \right)}^{10}}\approx 0.1%\] | \[\approx 99.9%\] |
| t = n (N1/2) | \[{{\left( \frac{1}{2} \right)}^{n}}\] | \[\left\{ 1-{{\left( \frac{1}{2} \right)}^{n}} \right\}\] |
(4) Mean (or average) life (t) : The time for which a radioactive material remains active is defined as mean (average) life of that material.
(i) or it is defined as the sum of lives of all atoms divided by the total number of atoms
i.e. \[\tau =\frac{\text{Sum of the lives of all the atoms}}{\text{Total number of atoms}}=\frac{1}{\lambda }\]
(ii) From \[N={{N}_{0}}{{e}^{-\lambda t}}\]Þ \[\frac{\ln \frac{N}{{{N}_{0}}}}{t}=-\lambda \] slope of the line shown in the graph i.e. the magnitude of inverse of slope of \[\ln \frac{N}{{{N}_{0}}}\,\,vs\,\,t\] curve is known as mean life \[(\tau )\].
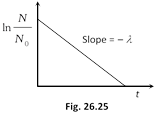
(iii) From \[N={{N}_{0}}{{e}^{-\lambda t}}\], if \[t=\frac{1}{\lambda }=\tau \]
\[\Rightarrow \] \[N={{N}_{0}}{{e}^{-1}}={{N}_{0}}\left( \frac{1}{e} \right)=0.37{{N}_{0}}=37%\] of N0.
i.e. mean life is the time interval in which number of undecayed atoms (N) becomes \[\frac{1}{e}\] times or 0.37 times or 37% of original number of atoms.
or
It is the time in which number of decayed atoms (N0 – N) becomes \[\left( 1-\frac{1}{e} \right)\] times or 0.63 times or 63% of original number of atoms.
(iv) From \[{{T}_{1/2}}=\frac{0.693}{\lambda }\] \[\Rightarrow \] \[\frac{1}{\lambda }=\tau =\frac{1}{0.693}.\,({{t}_{1/2}})=1.44\,({{T}_{1/2}})\]
i.e. mean life is about 44% more than that of half life. Which gives us \[\tau >{{T}_{(1/2)}}\]
You need to login to perform this action.
You will be redirected in
3 sec
