Continuous X-Rays
Category : JEE Main & Advanced
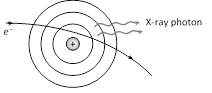
As an electron passes close to the positive nucleus of atom of the target, the electron is deflected from it's path as shown in figure. This results in deceleration of the electron. The loss in energy of the electron during deceleration is emitted in the form of X-rays.
The X-ray photons so emitted form the continuous X-ray spectrum.
(1) Minimum wavelength : When the electron looses whole of it's energy in a single collision with the atom, an X-ray photon of maximum energy h\[{{v}_{\max }}\] is emitted i.e.\[\frac{1}{2}m{{v}^{2}}=eV=h{{\nu }_{\max }}=\frac{hc}{{{\lambda }_{\min }}}\]
where v = velocity of electron before collision with target atom, V = potential difference through which electron is accelerated, c = speed of light \[=3\times {{10}^{8}}\,m/s\]
Maximum frequency of radiations (X-rays) \[{{\nu }_{\max }}=\frac{eV}{h}\]
Minimum wavelength = cut off wavelength of X-ray
\[{{\lambda }_{\min }}=\frac{hc}{eV}=\frac{12375}{V}{\AA}\]
(2) Intensity wavelength graph : The continuous X-ray spectra consist of all the wavelengths over a given range. These wavelength are of different intensities. Following figure shows the intensity variation of different wavelengths for various accelerating voltages applied to X-ray tube.
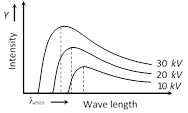
For each voltage, the intensity curve starts at a particular minimum wavelength \[({{\lambda }_{\min }})\]. Rises rapidly to a maximum and then drops gradually.
The wavelength at which the intensity is maximum depends on the accelerating voltage, being shorter for higher voltage and vice-versa.
You need to login to perform this action.
You will be redirected in
3 sec
