Answer:
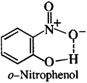
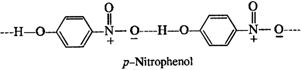
In o-nitrophenol, intra molecular hydrogen bonding is present while inter molecular hydrogen is present in the molecules of p-nitrophenol. Energy is needed to overcome attractive forces in the molecules of p-nitrophenol but no such energy is required in case of molecules in o-nitrophenol. This means that the boiling point of o-nitrophenol is less and is steam volatile while that of p-nitrophenol is more and is, therefore, non-volatile in steam.
You need to login to perform this action.
You will be redirected in
3 sec
