Answer:
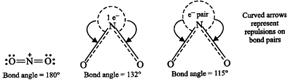
\[NO_{2}^{+}>N{{O}_{2}}>NO_{2}^{-}\]. This is because \[NO_{2}^{+}\] has no lone pair of electrons (i.e. has only bond pairs on two sides) and hence it is linear. \[N{{O}_{2}}\] has one unshared electron while \[NO_{2}^{-}\] has one unshared electron pair. There are greater repulsions on \[N-O\] bonds in case of \[NO_{2}^{-}\] than in case of \[N{{O}_{2}}\].
You need to login to perform this action.
You will be redirected in
3 sec
