Answer:
In chloral hydrate, the presence of
three \[Cl\] atoms with \[-I\] effect increases the magnitude of the positive
charge on the carbonyl carbon atom. As a result, even a weak nucleophile like \[{{H}_{2}}O\]
attacks to form a hydrate. Moreover, there is weak intermolecular hydrogen
bonding in the chloral hydrate which tends to increase its stability.
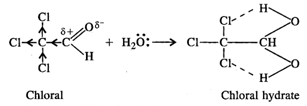 or \[CC{{l}_{3}}CH{{(OH)}_{2}}\]
or \[CC{{l}_{3}}CH{{(OH)}_{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
