Answer:
(a)\[\underset{\text{Acetylene}}{\mathop{HC\equiv
CH}}\,\xrightarrow[\text{(Addition}\,\text{of}\,{{\text{H}}_{\text{2}}}\text{O)}]{\text{dil}\text{.}{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{.}\,\text{HgS}{{\text{O}}_{\text{4}}}}\underset{\text{Vinyl}\,\,\text{alcohol}}{\mathop{[{{H}_{2}}C=CHOH]}}\,\]\[\xrightarrow{\text{Tautomerises}}\,\underset{\text{Acetaldehyde}\,\text{(A)}}{\mathop{C{{H}_{3}}-CHO}}\,\xrightarrow[\text{(Aldol}\,\,\text{condensation)}]{\text{dil}\text{.}\,\text{NaOH}}\]
\[\underset{\begin{smallmatrix}
\text{
}\!\!\beta\!\!\text{ -Hydroxybutyraldehyde}\,\text{(B)} \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(An\,aldol)
\end{smallmatrix}}{\mathop{C{{H}_{3}}-CHOH-C{{H}_{2}}-CHO}}\,\xrightarrow[\text{Dehydration}\,\text{(-}{{\text{H}}_{\text{2}}}\text{O)}]{\text{heat}}\]\[\underset{\text{But-2-en-1-al}\,\text{(C)}}{\mathop{C{{H}_{3}}-CH=CH-CHO}}\,\]
(b) (i) Higher the \[{{K}_{a}},\]
stronger is the acid. Thus, p-nitrobenzoic acid is a stronger acid than benzoic
acid. This is due to the following two reasons :
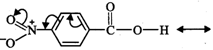
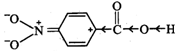
I. Due to -\[I\] and -R-effect
of the \[-N{{O}_{2}}\] group, the electron density in the \[O-H\] bond
decreases. As a result, \[O-H\] bond becomes weak and hence p-nitrobenzoic acid
more easily loses a proton than benzoic acid
 II. Due to -\[I\] and-R-effect
of the \[N{{O}_{2}}\] group, dispersal of the -ve charge occurs and hence
p-nitrobenzoate ion becomes more stable than benzoate ion.
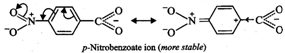
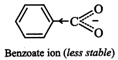
II. Due to -\[I\] and-R-effect
of the \[N{{O}_{2}}\] group, dispersal of the -ve charge occurs and hence
p-nitrobenzoate ion becomes more stable than benzoate ion.
 (ii) In acetone, C = O group
easily forms H-bonds with water and hence acetone is highly soluble in water. However,
in benzophenone, the phenyl groups are big and hence C = O group cannot form
H-bonds with water due to steric hindrance and hence benzophenone is insoluble
in water.
(ii) In acetone, C = O group
easily forms H-bonds with water and hence acetone is highly soluble in water. However,
in benzophenone, the phenyl groups are big and hence C = O group cannot form
H-bonds with water due to steric hindrance and hence benzophenone is insoluble
in water.
You need to login to perform this action.
You will be redirected in
3 sec
