Answer:
Amines are more basic than
comparable alcohols because of the following two reasons:
(i) N being less electronegative is more
willing to donate its lone pair of electrons to a proton than the more
electronegative O atom. Therefore, amines are more basic than alcohols.
(ii) When an amine accepts a
proton, ammonium salt is formed and when an alcohol accepts a proton oxonium
salt is formed.
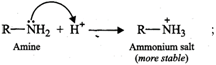
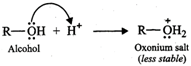 Since N being less
electronegative can accommodate the positive charge better than the more
electronegative O atom, therefore, ammonium salts are more stable than oxonium
salts. As a result, amines are more basic than alcohols.
Since N being less
electronegative can accommodate the positive charge better than the more
electronegative O atom, therefore, ammonium salts are more stable than oxonium
salts. As a result, amines are more basic than alcohols.
You need to login to perform this action.
You will be redirected in
3 sec
