Answer:
Due to \[+I\]-effect of the alkyl groups, the electron density on the N-atom of \[1{}^\circ ,\,2{}^\circ \] and \[3{}^\circ \] amines is higher than that on the N-atom in \[N{{H}_{3}}\]. Therefore, all amines are more basic than \[N{{H}_{3}}\].
(i) In gaseous phase, solvation effects are absent and hence the relative basicity of amines depends only on the \[+I\]-effect of the alkyl groups. Now since the \[+I\]-effect increases in going from \[1{}^\circ \] to \[2{}^\circ \] to \[3{}^\circ \]amine, therefore, the relative basicity of amines decreases in the order :
\[3{}^\circ \] amine > \[2{}^\circ \] amine > \[1{}^\circ \]amine. In other words, in the gaseous phase, the basicity of methylamines relative to \[N{{H}_{3}}\] decreases in the order :
\[{{(C{{H}_{3}})}_{3}}N>{{(C{{H}_{3}})}_{2}}NH>C{{H}_{3}}N{{H}_{2}}>N{{H}_{3}}\]
(ii) In aqueous solution, the basicity of methylammes depends upon two factors :
(a) \[+I\]-effect of the \[C{{H}_{3}}\] groups and
(b) Solvation effect, i.e., stabilization of the conjugate acid (formed by addition of a proton to amine) by H-bonding.
As explained above, on the basis of \[+I\]-effect alone, the relative basicity of amines should decrease in the order :\[{{(C{{H}_{3}})}_{3}}N>{{(C{{H}_{3}})}_{2}}NH>C{{H}_{3}}N{{H}_{2}}>N{{H}_{3}}\]
On the basis of stabilization of the conjugate acids by H- bonding alone (as explained below), the basicity should decrease in the order: \[C{{H}_{3}}N{{H}_{2}}>{{(C{{H}_{3}})}_{2}}NH>{{(C{{H}_{3}})}_{3}}N\]
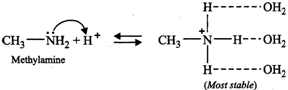
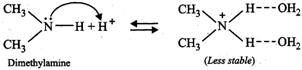
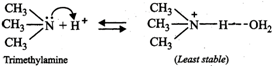 The combined effect of these two opposing factors is that \[{{(C{{H}_{3}})}_{2}}NH\] is the strongest base. In case of \[C{{H}_{3}}N{{H}_{2}}\] and \[{{(C{{H}_{3}})}_{3}}N,\] the stability due to H-bonding predominates over stability due to \[+I\]-effect of the\[C{{H}_{3}}\] groups thereby making \[C{{H}_{3}}N{{H}_{2}}\] stronger than \[{{(C{{H}_{3}})}_{3}}N\]. Thus, the overall relative basicity of methylamines w.r.t. ammonia in aqueous solution decreases in the order :
\[{{(C{{H}_{3}})}_{2}}NH>C{{H}_{3}}N{{H}_{2}}>{{(C{{H}_{3}})}_{3}}N>N{{H}_{3}}\]
The combined effect of these two opposing factors is that \[{{(C{{H}_{3}})}_{2}}NH\] is the strongest base. In case of \[C{{H}_{3}}N{{H}_{2}}\] and \[{{(C{{H}_{3}})}_{3}}N,\] the stability due to H-bonding predominates over stability due to \[+I\]-effect of the\[C{{H}_{3}}\] groups thereby making \[C{{H}_{3}}N{{H}_{2}}\] stronger than \[{{(C{{H}_{3}})}_{3}}N\]. Thus, the overall relative basicity of methylamines w.r.t. ammonia in aqueous solution decreases in the order :
\[{{(C{{H}_{3}})}_{2}}NH>C{{H}_{3}}N{{H}_{2}}>{{(C{{H}_{3}})}_{3}}N>N{{H}_{3}}\]
You need to login to perform this action.
You will be redirected in
3 sec
