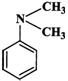
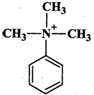
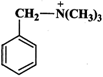
Answer:
Higher the electron density in the benzene ring, more reactive is the aromatic compound towards electrophilic substitution reaction. Now due to the presence of a lone pair of electrons on the N-atom which is can directly donate to the benzene ring, \[N{{(C{{H}_{3}})}_{2}}\] is a much stronger electron donating group than \[C{{H}_{3}}\] group. The remaining two groups contain a positive charge on the N-atom and hence act as electron-withdrawing groups. But in \[{{(C{{H}_{3}})}_{3}}\overset{+}{\mathop{N}}\,-\]group, the +vely charged N is directly attached to the benzene ring, therefore, its electron-withdrawing ability is much stronger than \[-C{{H}_{2}}\overset{+}{\mathop{N}}\,{{(C{{H}_{3}})}_{2}}\]. From the above discussion, it follows that the electron density in the benzene ring increases in the order :
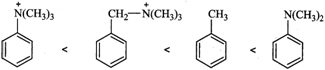 Therefore, their reactivity towards electrophilic substitution reactions also increases in the same order.
Therefore, their reactivity towards electrophilic substitution reactions also increases in the same order.
You need to login to perform this action.
You will be redirected in
3 sec
