Answer:
(a) Carbon and nitrogen atoms have
comparable sizes. They can be linked to each
other
by triple bond to form \[C\equiv \overset{\odot -}{\mathop{N}}\,:\]ion in which
\[{{p}_{\pi }}-{{p}_{\pi }}\] bonding is possible. However, similar \[{{p}_{\pi
}}-{{p}_{\pi }}\] bonding is not possible between carbon and phosphorus atoms
because of comparatively large atomic size of phosphorus. Therefore, \[C\equiv
\overset{\odot -}{\mathop{P}}\,:\] ion does not exist.
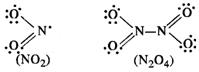
(b) \[N{{O}_{2}}\] is an odd electron
molecule and has 17 valence electrons (5 due to N and 12 due to two 0 atoms).
Upon dimerisation, it changes into a stable \[{{N}_{2}}{{O}_{4}}\] molecule
with even number of electrons (34).
 (c) \[\text{ICl}\] is polar \[(\overset{\,\delta
+}{\mathop{I}}\,-\overset{\delta -}{\mathop{C}}\,l)\] due to the electro negativity
difference between the two participating halogen atoms. It is therefore, more
reactive than 12 which is completely non-polar.
(c) \[\text{ICl}\] is polar \[(\overset{\,\delta
+}{\mathop{I}}\,-\overset{\delta -}{\mathop{C}}\,l)\] due to the electro negativity
difference between the two participating halogen atoms. It is therefore, more
reactive than 12 which is completely non-polar.
You need to login to perform this action.
You will be redirected in
3 sec
