Answer:
For a circular orbit of the electron, \[\frac{m{{\upsilon }^{2}}}{r}=\frac{kZe.e}{{{r}^{2}}}=\frac{kZ{{e}^{2}}}{{{r}^{2}}}\] or \[m{{\upsilon }^{2}}=\frac{kZ{{e}^{2}}}{r}\] or \[r=\frac{kZ{{e}^{2}}}{m{{\upsilon }^{2}}}\] ? (i) Using Bohr?s quantisation condition for angular momentum, \[L=m\upsilon r=\frac{nh}{2\pi }\] or \[r=\frac{nh}{2\pi m\upsilon }\] ?(ii) From (i) and (ii), \[\frac{kZ{{e}^{2}}}{m{{\upsilon }^{2}}}=\frac{nh}{2\pi m\upsilon }\] or \[\upsilon =\frac{2\pi kZ{{e}^{2}}}{nh}\] \[\therefore \] \[r=\frac{nh}{2\pi m}.\frac{nh}{2\pi kZ{{e}^{2}}}\] \[=\frac{{{n}^{2}}{{h}^{2}}}{4{{\pi }^{2}}mkZ{{e}^{2}}}\] As \[r\propto {{n}^{2}},\]the graph of r versus n is a parabola as shown in Fig. 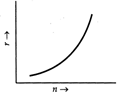
You need to login to perform this action.
You will be redirected in
3 sec
