Answer:
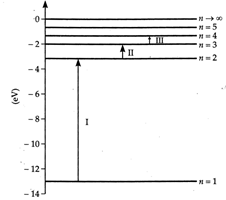
(i) Line \[I\to \] Lyman series. Line \[II\to \] Balmer series and Line \[III\to \] Paschen series (ii) Line \[III\] corresponds to absorption of photon of minimum energy and hence of maximum wavelength.
You need to login to perform this action.
You will be redirected in
3 sec
