A) Absence of a-hydrogen
B) Resonance stabilisation of carboxylate ion
C) Reactivity of a-hydrogen
D) Hydrogen bond
Correct Answer: B
Solution :
Carboxylic acids are easily ionized because there is resonance in carboxylate ion due to p- electron shifting so \[{{H}^{+}}\] get ionised very easily.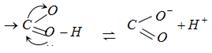
You need to login to perform this action.
You will be redirected in
3 sec
