A) Square planar
B) Tetrahedral
C) Pyramidal
D) Pentagonal
Correct Answer: A
Solution :
Since hybridisation is \[ds{{p}^{2}}\]so it is square planar,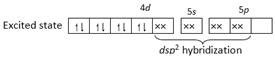
You need to login to perform this action.
You will be redirected in
3 sec
