A) m-bromophenol
B) o-and p-bromophenol
C) p-bromophenol
D) 2, 4, 6-tribromophenol
Correct Answer: B
Solution :
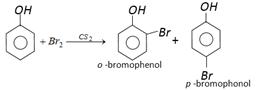 In presence of non-polar solvent \[(C{{S}_{2}})\] the ionization of phenol is suppressed. The ring is slightly activated and hence mono substitution occurs. On the other hand with \[B{{r}_{2}}\] water phenol forms 2, 4, 6-tribromo phenol.
In presence of non-polar solvent \[(C{{S}_{2}})\] the ionization of phenol is suppressed. The ring is slightly activated and hence mono substitution occurs. On the other hand with \[B{{r}_{2}}\] water phenol forms 2, 4, 6-tribromo phenol. 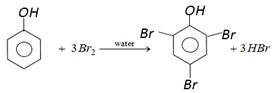 In aqueous solution phenol ionizes to give phenoxide ion. Due to the presence of negative charge on oxygen the benzene ring is highly activated and hence trisubstituted product is obtained.
In aqueous solution phenol ionizes to give phenoxide ion. Due to the presence of negative charge on oxygen the benzene ring is highly activated and hence trisubstituted product is obtained.
You need to login to perform this action.
You will be redirected in
3 sec
