Answer:
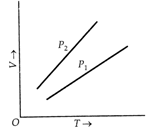
For a perfect gas, \[PV=nRT\] \[\therefore \] \[V=\frac{nR}{P}T\] \[\therefore \] Slope of \[V-T\]graph with \[\text{T- axis}\] \[=\frac{nR}{P}\] For a given amount of gas, slope \[\propto \frac{1}{P}\]
You need to login to perform this action.
You will be redirected in
3 sec
