Answer:
(i) ![]() is
more acidic than
is
more acidic than ![]() As
we go down the group, electro negativity decreases hence stability of (H?X)
bond decreases. Thus tendency of the (H?X) to release
As
we go down the group, electro negativity decreases hence stability of (H?X)
bond decreases. Thus tendency of the (H?X) to release![]() increase.
Thus,
increase.
Thus, ![]() with weak
(H?S) bond is more acidic than
with weak
(H?S) bond is more acidic than ![]()
![]()
![]() [1]
(ii)
[1]
(ii)
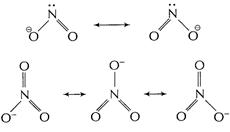 In
In ![]() two resonating
structures and in
two resonating
structures and in ![]() three
resonating structures are formed. Thus (N?O) bond length in
three
resonating structures are formed. Thus (N?O) bond length in ![]() is
shorter than that in
is
shorter than that in ![]() .
(iii)
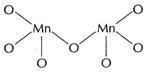
Fluorine is the most electronegative element with valency (-1). It stabilises
the metal ion by single bond formation as in
.
(iii)
Fluorine is the most electronegative element with valency (-1). It stabilises
the metal ion by single bond formation as in![]()
![]() Oxygen
forms multiple bonds with metals and stabilises the compound to a greater
extent.
Oxygen
forms multiple bonds with metals and stabilises the compound to a greater
extent.
![]() is stable
due to multiple bonds.
is stable
due to multiple bonds.
 [1]
[1]
You need to login to perform this action.
You will be redirected in
3 sec
