Answer:
(a) (i)![]()
 (ii)
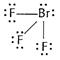
(ii) ![]() is
interhalogen compound Br and F both complete their octets.
is
interhalogen compound Br and F both complete their octets.
 (b)
(b) ![]() [1]
(ii)
[1]
(ii) ![]() [1]
(iii)
[1]
(iii)![]()
![]() [1]
Or
(a) (i)
[1]
Or
(a) (i)![]()
![]() [1]
(ii) FE
(III) is reduced to Fe (II)
[1]
(ii) FE
(III) is reduced to Fe (II)
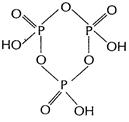
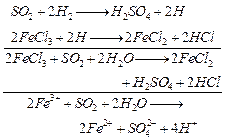 (b) (i)
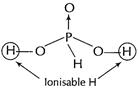
(b) (i) ![]() has two ion
sable H and is thus it is a dibasic acid.
has two ion
sable H and is thus it is a dibasic acid.
 (ii) Because
F is the most electronegative element of the halogen family as well as in the
whole Periodic Table. It only forms
(ii) Because
F is the most electronegative element of the halogen family as well as in the
whole Periodic Table. It only forms![]() and not
and not![]() . Thus it
is never as the central atom in interhalogen compounds. Also it lacks d-orbital
in which electrons can be expanded to give valency more than 1. Cl, Br, I with
d-orbital can expand its valency to +1,+3,+5,+7
[1]
(iii) They
have only weak dispersion forces being monoatomic in nature. Thus, they have
low boiling points. [1]
. Thus it
is never as the central atom in interhalogen compounds. Also it lacks d-orbital
in which electrons can be expanded to give valency more than 1. Cl, Br, I with
d-orbital can expand its valency to +1,+3,+5,+7
[1]
(iii) They
have only weak dispersion forces being monoatomic in nature. Thus, they have
low boiling points. [1]
You need to login to perform this action.
You will be redirected in
3 sec
