Answer:
(a)
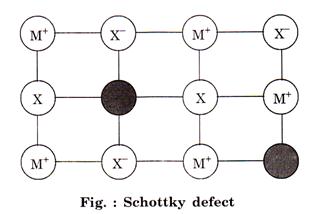
Schottky defect: This type of defect is created when one positive ion and
one negative hp ion are missing from their respective positions
learning behind a pair of holes. 1
These are more common in ionic compounds with high
co-ordination number, e.g.,![]()
![]()
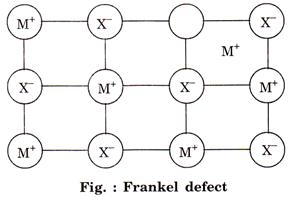
![]() (b) Frankel defect: This defect is created when an
ion leaves its correct lattice and occupies an interstitial site. There is no
change in the density of crystal, e.g.,
(b) Frankel defect: This defect is created when an
ion leaves its correct lattice and occupies an interstitial site. There is no
change in the density of crystal, e.g., ![]() .
.
 1
1
You need to login to perform this action.
You will be redirected in
3 sec
