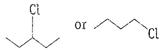 Or
Write the structures and names of the compounds formed
when compound A with molecular formula
Or
Write the structures and names of the compounds formed
when compound A with molecular formula Answer:
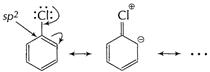
In haloarenes (C?Cl) bond is stabilised by resonance. It
acquires certain double bond character.
Also it is attached to ![]() -hybridised
carbon.
-hybridised
carbon.
 In haloalkanes, resonance stabilisation" of (C?Cl) bond
does not take place. Also Cl is attached to
In haloalkanes, resonance stabilisation" of (C?Cl) bond
does not take place. Also Cl is attached to ![]() -hybridised
carbon which is less electronegative than
-hybridised
carbon which is less electronegative than ![]() -hybridised
carbon in haloarenes.
-hybridised
carbon in haloarenes.
![]() Thus, haloalkanes are more reactive for
Thus, haloalkanes are more reactive for ![]() reactions
than haloarenes.
(ii)
reactions
than haloarenes.
(ii)
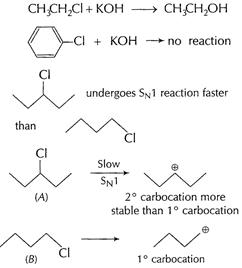 [1]
In (A),
[1]
In (A),![]() carbocation
formed is more stable than
carbocation
formed is more stable than ![]() carbocation
from (B). [1]
Or
carbocation
from (B). [1]
Or
You need to login to perform this action.
You will be redirected in
3 sec
