Answer:
(a) (i) Phosphorus exists as ?4 with structure as shown
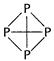 Nitrogen exists as N; with structure N =N
BE of (P?P) bonds in
Nitrogen exists as N; with structure N =N
BE of (P?P) bonds in ![]() is 215
kJ
is 215
kJ ![]() while BE
of (N = N) bond in N;, is 946 kJ
while BE
of (N = N) bond in N;, is 946 kJ ![]() . Greater
the BE greater the stability and smaller the reactivity.
Thus, nitrogen is very less reactive than phosphorus.
[1]
(ii) H?F molecules are joined by intermolecular H-bond
such that each molecule is joined at two points by other H?F molecules.
. Greater
the BE greater the stability and smaller the reactivity.
Thus, nitrogen is very less reactive than phosphorus.
[1]
(ii) H?F molecules are joined by intermolecular H-bond
such that each molecule is joined at two points by other H?F molecules.
![]() In case of
In case of ![]() , each
molecule is joined to four water molecules.
, each
molecule is joined to four water molecules.
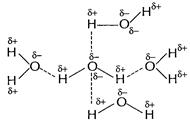 Hence, larger heat is required to break H-bonding in
Hence, larger heat is required to break H-bonding in ![]() molecules
to convert them into vapours as compared to that in H?F molecules. Hence, H?F boils
at lower temperature than
molecules
to convert them into vapours as compared to that in H?F molecules. Hence, H?F boils
at lower temperature than ![]() .
[1]
(iii)
.
[1]
(iii) ![]() Oxygen has no d-orbital, hence unpaired electrons cannot
be excited to expand its valency beyond two.
Thus, oxygen exists as
Oxygen has no d-orbital, hence unpaired electrons cannot
be excited to expand its valency beyond two.
Thus, oxygen exists as ![]() .
. ![]()
![]() Sulphur has vacant d-orbital, thus it can expand its valency
beyond two.
Also (BE) of (S?S) bond in S8 is smaller than
that of (O=O) in
Sulphur has vacant d-orbital, thus it can expand its valency
beyond two.
Also (BE) of (S?S) bond in S8 is smaller than
that of (O=O) in ![]() .
Thus, catenation in sulphur is larger than that in oxygen [1]
(b) (i)
.
Thus, catenation in sulphur is larger than that in oxygen [1]
(b) (i)
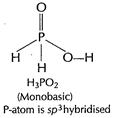 [1]
(ii)
[1]
(ii)
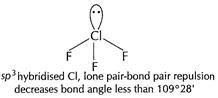 [1]
Or
(a) (i)
[1]
Or
(a) (i) ![]()
![]() As we go down the group, electro negativity decreases BE
of (H?S) bond in
As we go down the group, electro negativity decreases BE
of (H?S) bond in ![]() is
smaller than that of (O?H) bond in
is
smaller than that of (O?H) bond in ![]() . Thus, ionization
of
. Thus, ionization
of ![]() . Is
larger than that of
. Is
larger than that of![]() .
Thus,
.
Thus, ![]() is more
acidic than
is more
acidic than![]() [1]
(ii) Fluorine is the most electronegative element of the Periodic
Table.
[1]
(ii) Fluorine is the most electronegative element of the Periodic
Table.
![]() It can gain electron to form stable Ne configuration
It can gain electron to form stable Ne configuration ![]() . There
is no d-orbital in which electron can be excited to form positive oxidation
state.
. There
is no d-orbital in which electron can be excited to form positive oxidation
state.
![]()
![]() [1]
Thus, F shows only negative (-1) oxidation state.
(ii) He (l52)
Most stable and with maximum ionisation potential.
Thus, it does not form
[1]
Thus, F shows only negative (-1) oxidation state.
(ii) He (l52)
Most stable and with maximum ionisation potential.
Thus, it does not form ![]() or
or ![]() [1]
(b) (i)
[1]
(b) (i)
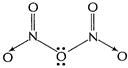 (ii)
(ii)
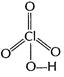
You need to login to perform this action.
You will be redirected in
3 sec
