Answer:
Haloarenes are much less reactive than haloalkanes towards![]() reactions
due to the following reasons
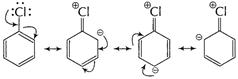
(i) Resonance effect (C?Cl) bond is stabilised due to
resonance. This resonance arises as a result of delocalisation of lone pair on Cl
atoms and
reactions
due to the following reasons
(i) Resonance effect (C?Cl) bond is stabilised due to
resonance. This resonance arises as a result of delocalisation of lone pair on Cl
atoms and ![]() -elctrons
in benzene sextet. (C?Cl) bond acquires partial double bond character.
-elctrons
in benzene sextet. (C?Cl) bond acquires partial double bond character.
![]() (ii)
(ii)
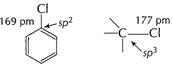 Electronegativity of sp2 > sp3
(C?Cl) bond length in aryl halide < alkyi halide Bond energy (C?Cl) > alkyi
halide, Thus, sn reaction is not
feasible in aryl halide.
Electronegativity of sp2 > sp3
(C?Cl) bond length in aryl halide < alkyi halide Bond energy (C?Cl) > alkyi
halide, Thus, sn reaction is not
feasible in aryl halide. ![]() (iii) If
(iii) If ![]() reaction
takes place, phenyl carbocation isvery less stable than alkyl
carbocation.
reaction
takes place, phenyl carbocation isvery less stable than alkyl
carbocation. ![]() (iv) Nucleophile is repelled by electron rich aryl halide.
Or
(i) For
(iv) Nucleophile is repelled by electron rich aryl halide.
Or
(i) For ![]() reaction
reaction
![]() [1]
[1]
![]() being
larger in size causes steric hindrance for nucleophilic attack.
(ii)
being
larger in size causes steric hindrance for nucleophilic attack.
(ii) 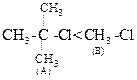 Due to the presence of three alky] groups in (A), nucleophile
cannot approach central carbon in
Due to the presence of three alky] groups in (A), nucleophile
cannot approach central carbon in ![]() reaction.
Thus,
reaction.
Thus, ![]() reaction
is faster in (6).
reaction
is faster in (6).
You need to login to perform this action.
You will be redirected in
3 sec
