Answer:
(i) ![]() Oxidation
number of Cr = 0
Cr (24) [Ar] 3d5 4s1
Oxidation
number of Cr = 0
Cr (24) [Ar] 3d5 4s1
![]() Cr (when complexing with CO)
Cr (when complexing with CO)
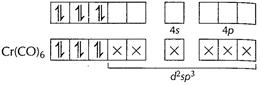
![]() Electrons
donated by ligands CO. Hybridisation d3sp3 Inner
d-orbital complex Unpaired electron = zero
Thus, diamagnetic in nature. [1]
(ii)
Electrons
donated by ligands CO. Hybridisation d3sp3 Inner
d-orbital complex Unpaired electron = zero
Thus, diamagnetic in nature. [1]
(ii)
For a given ion and ligand, greater the charge on the metal ion, greater
the stability. Thus,
![]()
![]()
Ligand
![]() CN 6
ON of Fe +2
CN 6
ON of Fe +2
![]() 6
+3
6
+3
![]() [1]
(iii) Various factor affecting crystal field splitting Nature
of the ligand Which have been arranged in increasing field strength as
[1]
(iii) Various factor affecting crystal field splitting Nature
of the ligand Which have been arranged in increasing field strength as
![]()
![]() (b) Geometry of the complex
(b) Geometry of the complex ![]() values (of
tetrahedral complex) is about 50% compared to
values (of
tetrahedral complex) is about 50% compared to ![]() value of octahedral complex).
(c) Nature of metal ion
3d :high spin 4d,5d,: low spin
(d) Oxidation state of metal ion
value of octahedral complex).
(c) Nature of metal ion
3d :high spin 4d,5d,: low spin
(d) Oxidation state of metal ion
![]()
![]() [1]
Thus, greater the charge on metal ion, greater the value
of
[1]
Thus, greater the charge on metal ion, greater the value
of ![]() .
.
You need to login to perform this action.
You will be redirected in
3 sec
