Answer:
An ideal solution consisting of two components A and B is
one in which the intermediate attractions A...A, B...B and A...B are
equal.
For such cases ![]() In such cases, there is no deviation from Raoult's law
In such cases, there is no deviation from Raoult's law ![]() If A..B attractions are smaller than the average A...A
and B...B attractions then the vapour pressure of the mixture is
larger than the expected value. For such cases
If A..B attractions are smaller than the average A...A
and B...B attractions then the vapour pressure of the mixture is
larger than the expected value. For such cases ![]() (endothermic)
This is called positive deviation from Raoult's
law. [1]
Example Ethanol and acetone form solution with positive
deviation.
If A...B attractions are greater than the average
of A...A and B...B attraction, then vapour pressure of the mixture is
less than the expected value. For such case
(endothermic)
This is called positive deviation from Raoult's
law. [1]
Example Ethanol and acetone form solution with positive
deviation.
If A...B attractions are greater than the average
of A...A and B...B attraction, then vapour pressure of the mixture is
less than the expected value. For such case![]() (exothermic)
This is called negative deviation from Raoult's
law.
Example Acetone and chloroform mixture [1]
(exothermic)
This is called negative deviation from Raoult's
law.
Example Acetone and chloroform mixture [1]
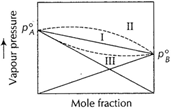 I Ideal
II Positive deviation
III Negative deviation
I Ideal
II Positive deviation
III Negative deviation
You need to login to perform this action.
You will be redirected in
3 sec
