Answer:
![]() is
hydrolysed to a greater extent as compared to
is
hydrolysed to a greater extent as compared to![]()
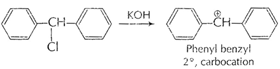 [1]
This is more stable due to resonance because of
delocalization of
[1]
This is more stable due to resonance because of
delocalization of ![]() -electrons
in two benzene ring.
-electrons
in two benzene ring.
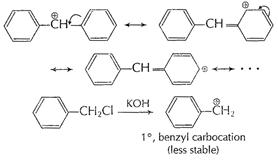 Or
Aqueous KOH is a strong base ionising completely and thus
releasing free
Or
Aqueous KOH is a strong base ionising completely and thus
releasing free ![]() ions.
This displace halogen from alkyl halides in
ions.
This displace halogen from alkyl halides in ![]() reaction.
reaction.
![]()
![]() [1]
When alcoholic KOH is taken, then alkoxide
[1]
When alcoholic KOH is taken, then alkoxide ![]() is formed.
is formed.
![]() is a
stronger base than
is a
stronger base than ![]() and
causes dehvdrohalogenation (loss of H X) from alkyl halide.
and
causes dehvdrohalogenation (loss of H X) from alkyl halide.
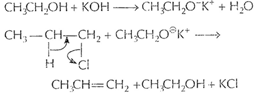
You need to login to perform this action.
You will be redirected in
3 sec
