Answer:
D-glucose exists in two anomeric forms, in cyclic forms.
Open structure has following drawbacks
(i) It does not form addition compounds with ![]() and
Grignard reagent thus, indicating absence of ?CHO group. [1]
(ii) It does not give Schiff's test. [1]
(iii) Pent acetate of glucose does not react with hydroxyl
ammine. [1]
All these facts can be explained if we take cyclic structure.
and
Grignard reagent thus, indicating absence of ?CHO group. [1]
(ii) It does not give Schiff's test. [1]
(iii) Pent acetate of glucose does not react with hydroxyl
ammine. [1]
All these facts can be explained if we take cyclic structure.
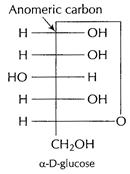
![]() glucose
differs in configuration about numeric carbon.
glucose
differs in configuration about numeric carbon.
You need to login to perform this action.
You will be redirected in
3 sec
