Answer:
(i) When peptising agent is added to freshly prepared precipitate,
it changes into colloidal sol. It is called peptisation. During peptisation,
the precipitate adsorbs one of the ions of the electrolyte on its surface.
Thus, there is formation of positive or negative charge on the precipitate which
ultimately break up into smaller particles of the size of the colloidal sol.
Agl is precipitated in the following reaction
![]() If Kl is added to Agl ppt,
If Kl is added to Agl ppt, ![]() (common
ion) is adsorbed on this surface and negatively-charged colloidal sol is
formed.
(common
ion) is adsorbed on this surface and negatively-charged colloidal sol is
formed.
![]() [1]
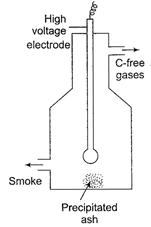
(ii) Smoke is a colloidal sol of solid particles such as carbon,
arsenic, dust dispersed in air. They are charged particles.
The smoke before it comes out from the chimney is led
through the chamber containing plates having a charge opposite to that carried
by smoke particles. (This chamber is called Cottrell precipitator). The
particles, on coming in contact with these plates lose their charge and get precipitated.
[1]
(ii) Smoke is a colloidal sol of solid particles such as carbon,
arsenic, dust dispersed in air. They are charged particles.
The smoke before it comes out from the chimney is led
through the chamber containing plates having a charge opposite to that carried
by smoke particles. (This chamber is called Cottrell precipitator). The
particles, on coming in contact with these plates lose their charge and get precipitated.
 [1]
(iii) Colloidal gold (in the form of medicine) are more effective
because they have large surface area and therefore, easily assimilated. Thus,
colloidal gold sol is used for intramuscular injection. [1]
[1]
(iii) Colloidal gold (in the form of medicine) are more effective
because they have large surface area and therefore, easily assimilated. Thus,
colloidal gold sol is used for intramuscular injection. [1]
You need to login to perform this action.
You will be redirected in
3 sec
