Answer:
![]()
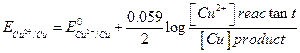 1
And
1
And
![]() ,
,
![]()
![]()
![]()
![]() 1
If
the concentration of
1
If
the concentration of ![]() ions
is decreased, the e.m.f. for copper electrode decreases.
ions
is decreased, the e.m.f. for copper electrode decreases. ![]()
You need to login to perform this action.
You will be redirected in
3 sec
