question_answer 1) A transverse wave passes through a string with the equation \[y=10\,\sin \,\pi \,(0.02\,x-2.00\,t)\]\[F=\frac{G{{m}_{1}}{{m}_{2}}}{{{r}^{2}}}\] where x is in metre and t in second. The maximum velocity of the particle in wave motion is:
A)
100 m/s
done
clear
B)
63m/s
done
clear
C)
120 m/s
done
clear
D)
161 m/s
done
clear
View Answer play_arrow
question_answer 2) A doctor prescribes spectacles to a patient with a combination of a convex lens of focal length 40 cm and convex lens of focal length 25 cm then the power of spectacles will be:
A)
-6.5D
done
clear
B)
1.5D
done
clear
C)
-1.5D
done
clear
D)
-8.5D
done
clear
View Answer play_arrow
question_answer 3) Parricles nature and wave nature of electromagnetic waves and electrons can be represented by :
A)
photelecmcity and electron microscopy
done
clear
B)
light is refracted and diffracted
done
clear
C)
X-rays is diffracted, reflected by thick metal sheet
done
clear
D)
electrons have small mass, deflected by the metal sheet
done
clear
View Answer play_arrow
question_answer 4) Same length of two identical wires are first connected is series and then in parallel, then the amount of heat produced in both the conditions are in the ratio:
A)
1 : 4
done
clear
B)
4 : 1
done
clear
C)
3 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 5) The ratio of intensities of two waves is 9 : 1. If they superimpose, the ratio of maximum to minimum intensity will be:
A)
3 : 1
done
clear
B)
4 : 9
done
clear
C)
4 : 1
done
clear
D)
1 : 9
done
clear
View Answer play_arrow
question_answer 6) Minimum number of 8\[{{m}_{1}}{{m}_{2}}\] and 250 V capacitors are used to make a combination of \[\therefore \] and 1000 V are :
A)
4
done
clear
B)
32
done
clear
C)
8
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 7) Let \[{{F}_{1}}={{F}_{2}}\] be the electric field due to a dipole in its axial plane distant \[\frac{G\left( \frac{M}{81} \right)M}{{{x}^{2}}}=\frac{GM\times M}{{{(60R-x)}^{2}}}\]and let \[\Rightarrow \] be the field in the equatorial plane distant \[\frac{1}{81{{x}^{2}}}=\frac{1}{{{(60R-x)}^{2}}}\] then the relatic between \[\frac{1}{9x}=\frac{1}{60R-x}\] and \[\Rightarrow \] will be:
A)
\[9x=60R-x\]
done
clear
B)
\[\Rightarrow \]
done
clear
C)
\[x=6R\]
done
clear
D)
\[{{\lambda }_{m}}T=constant\]
done
clear
View Answer play_arrow
question_answer 8) Given a current carrying wire of non-uniform cross-section. Which one of the following constant throughout the length of wire?
A)
Current only
done
clear
B)
Current and drift speed
done
clear
C)
Drift speed only
done
clear
D)
Current, electric field and drift speed
done
clear
View Answer play_arrow
question_answer 9) An insulated charged sphere of radius 5 cm has a potential of 10 V at the surface. The potential at the centre will be:
A)
same as that at 5 cm from the surface
done
clear
B)
same as that at 25 cm from the surface
done
clear
C)
10 V
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 10) Which one of the following is true?
A)
Momentum is conserved in all collisions but kinetic energy is conserved in elastic collisions
done
clear
B)
Momentum is not conserved in all collisions but kinetic energy is conserved in all collisions
done
clear
C)
Both momentum and kinetic energy an conserved in all collisions
done
clear
D)
Neither momentum nor kinetic energy is conserved in elastic collisions
done
clear
View Answer play_arrow
question_answer 11)
Match the items in list-I with items in list-II and collect the correct answers from the code given below the lists:
List-I
List-II
I. Myopia
A. Bifocal lens
II. Hyper-metropia
B. Cylindrical lens
III. Presbyopia
C. Concave lens
IV. Asrigmation
D. Convex lens
A)
\[I-D,\,\,II-C,\,\,III-A,\,\,IV-B\]
done
clear
B)
\[I-C,\,\,II-D,\,\,III-A,\,\,IV-B\]
done
clear
C)
\[I-B,\,\,II-D,\,\,III-A,\,\,IV-C\]
done
clear
D)
\[I-A,\,\,II-B,\,\,III-C,\,\,IV-D\]
done
clear
View Answer play_arrow
question_answer 12) Knowing that the mass of die moon is 1/81 limes that of earth and its radius is 1/4 the radius of earth. It the escape velocity at the surface of the earth is 11.2 km/s, then the value of escape velocity at the surface of the moon is:
A)
2.5 km/s
done
clear
B)
0.14 km/s
done
clear
C)
5 km/s
done
clear
D)
8 km/s
done
clear
View Answer play_arrow
question_answer 13) A body of mass 5 kg has momentum of 10 kg m/s, Mien a force of 0.2 N is applied on it for 10s the change in its kinetic , energy is:
A)
1.4 J
done
clear
B)
3.5 J
done
clear
C)
5.5 J
done
clear
D)
1.1 J
done
clear
View Answer play_arrow
question_answer 14) A body is allowed slide down a frictionless track freely under gravity. The track end in a semicircular shaped part of a diameter D. The height (minimum) from which the body must fall so that it completes the circle, is:
A)
\[\frac{4}{5}D\]
done
clear
B)
\[\frac{D}{2}\]
done
clear
C)
\[\frac{3D}{4}\]
done
clear
D)
\[\frac{5}{4}D\]
done
clear
View Answer play_arrow
question_answer 15) Half-life of a substance is 20 min, then the time between 33% decay and 67% decay will be:
A)
20 min
done
clear
B)
40 min
done
clear
C)
50 min
done
clear
D)
10 min
done
clear
View Answer play_arrow
question_answer 16) When a ray of light enters a glass slab, then:
A)
its frequency and wavelength changes
done
clear
B)
its frequency does not change
done
clear
C)
only frequency changes
done
clear
D)
its frequency and velocity changes
done
clear
View Answer play_arrow
question_answer 17) For an electron in the second orbit of hydrogen, me moment of momentum as per Bohrs model is :
A)
\[I=\frac{E}{R}=\frac{3}{30}=0.1A\]
done
clear
B)
\[U=\frac{{{q}_{1}}{{q}_{2}}}{4\pi {{\varepsilon }_{0}}}\left( \frac{1}{{{R}_{1}}}-\frac{1}{{{R}_{2}}} \right)\]
done
clear
C)
\[=9\times {{10}^{9}}\times 1\times {{10}^{-6}}\times {{10}^{-3}}\left( \frac{1}{1}-\frac{1}{10} \right)\]
done
clear
D)
\[=8.1J\]
done
clear
View Answer play_arrow
question_answer 18) What is the dimensional formula of gravitational constant?
A)
\[KE=\frac{1}{2}m{{v}^{2}}=8.1J\]
done
clear
B)
\[\Rightarrow \]
done
clear
C)
\[v=\sqrt{\frac{2\times 8.1}{2\times {{10}^{-3}}}}=90m/s\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 19) If work done in increasing the size of a soap film from 1C cm x 6 cm to 60 cm x 11 cm is\[F=eE\] what is the surface tension?
A)
\[=\frac{force}{mass}=\frac{eE}{m}\]
done
clear
B)
\[{{v}^{2}}={{u}^{2}}+2ax\]
done
clear
C)
\[u=0\]
done
clear
D)
Nope of these
done
clear
View Answer play_arrow
question_answer 20) A wave is represented by the equation \[\therefore \] where a and y are in cm and r in second. Velocity of propagation of [he wave is:
A)
200cm/s
done
clear
B)
10cm/s
done
clear
C)
25 cm/s
done
clear
D)
50 cm/s
done
clear
View Answer play_arrow
question_answer 21) If the mass of moon is \[{{v}^{2}}=2\left( \frac{eE}{m} \right)x\]. where M is the mass of earth, find the distance of the point from the moon. where gravitation field due to earth and moon cancel each other. Given that distance between earth and moon is 60R, where R is the radius of earth.
A)
4R
done
clear
B)
8 R
done
clear
C)
2R
done
clear
D)
6R
done
clear
View Answer play_arrow
question_answer 22) The sun emirs a light with maximum wave length 510 nm while another star emits a light with maximum wavelength of 350 nm. The ratio of surface temperature of sun and the star will be:
A)
0.68
done
clear
B)
2.1
done
clear
C)
1.45
done
clear
D)
0.46
done
clear
View Answer play_arrow
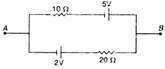
question_answer 23)
The current in the given circuit is:
A)
0.3 A
done
clear
B)
0.4 A
done
clear
C)
0.1 A
done
clear
D)
0.2 A
done
clear
View Answer play_arrow
question_answer 24) In a full wave rectifier circuit operating from 50 Hz mains frequency, then the fundamental frequency in the ripple will be:
A)
50 Hz
done
clear
B)
100Hz
done
clear
C)
70 Hz
done
clear
D)
25 Hz
done
clear
View Answer play_arrow
question_answer 25) A particle of mass 2g and charge \[E=\frac{V}{x}\]C is held at a distance of 1m from a fixed charge 1mC. If the particle is released it will be repelled. The speed of particle when it is at a distance of 10 m from the fixed charge is :
A)
90 m/s
done
clear
B)
100 m/s
done
clear
C)
45 m/s
done
clear
D)
55 m/s
done
clear
View Answer play_arrow
question_answer 26) An electron of mass m and charges e accelerated from rest through a potential difference V in vacuum. The final speed will be:
A)
\[\therefore \]
done
clear
B)
\[{{v}^{2}}=\frac{2eV}{m}\]
done
clear
C)
\[\Rightarrow \]
done
clear
D)
\[v=\sqrt{\frac{2eV}{m}}\]
done
clear
View Answer play_arrow
question_answer 27) Turn ratio in a step up transformer is 1 : 2, if a Lectanche cell of 1.5 V is connected across the input then the voltage across the output will be:
A)
0.1 V
done
clear
B)
1.5V
done
clear
C)
0.75 V
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 28) Which one of the following statement is not correct about the magnetic field?
A)
Inside the magnet the lines go from North Pole to South Pole of the magnet
done
clear
B)
Tangents to the magnetic lines give the direction of the magnetic field
done
clear
C)
The magnetic lines form a closed loop
done
clear
D)
Magnetic lines of force do not cut each other
done
clear
View Answer play_arrow
question_answer 29) What should be amount of current through the ring of radius of 5 cm so that field at the centre is equal to the earths magnetic field \[=eV\] is?
A)
0.28 A
done
clear
B)
5.57 A
done
clear
C)
2.8 A
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 30) A light of intensity \[{{I}_{0}}\] passes through a material of thickness d, then the intensity will be:
A)
\[\Rightarrow \]
done
clear
B)
\[\frac{1}{2}m{{v}^{2}}=eV\]
done
clear
C)
\[\Rightarrow \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 31)
A)
NAND gate
done
clear
B)
OR gate
done
clear
C)
AND gate
done
clear
D)
NOT gate
done
clear
View Answer play_arrow
question_answer 32) Assertion: Energy is released in nuclear fission. Reason: Total binding energy of the fission fragments is larger than the total binding energy of the parent nucleus.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 33) If each fission of 92U235releases 200 MeV, how many fissions must occur per second to produce power of 1 RW?
A)
\[v=\sqrt{\frac{2eV}{m}}\]
done
clear
B)
\[\pi \]
done
clear
C)
\[\mu F\]
done
clear
D)
\[16\mu F\]
done
clear
View Answer play_arrow
question_answer 34) Light of wavelength 589.3 nm is incident normally on a slit of width 0.1 mm. The angular width of the central diffraction maximum ac a distance of 1 m from the slit, is:
A)
0.68°
done
clear
B)
0.34°
done
clear
C)
2.05°
done
clear
D)
none of these
done
clear
View Answer play_arrow
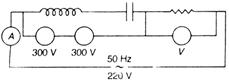
question_answer 35)
In the circuit shown below what will be the reading of the voltmeter and ammeter? (Total impedance of circuit Z = 100\[{{E}_{a}}\])
A)
200 V, 1A
done
clear
B)
800 V, 2A
done
clear
C)
100 V, 2A
done
clear
D)
220 V, 2.2A
done
clear
View Answer play_arrow
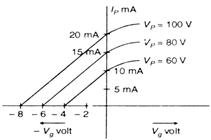
question_answer 36)
The variation of anode current in a triode value corresponding to a change in grid potential at three different values of the plate potential is shown in the given figure. The mutual conductance of triode is :
A)
\[l\]
done
clear
B)
\[{{E}_{q}}\]
done
clear
C)
\[l,\]
done
clear
D)
\[{{E}_{a}}\]
done
clear
View Answer play_arrow
question_answer 37) A ball of mass 10 kg is moving with vela of 10 m/s. It strikes another ball of mass 5kg which is moving in the same direction with a velocity of 4 m/s. If the collision is elastic their velocities after collision will be respectively:
A)
12 m/s, 6 m/s
done
clear
B)
12 m/s, 25 m/s
done
clear
C)
6 m/s, 12 m/s
done
clear
D)
8 m/s, 20 m/s
done
clear
View Answer play_arrow
question_answer 38) A body is released from the top of the tower metre high. It takes t second to reach d ground. Where is the body after t/2 second release?
A)
At 3H/4 metre from the ground
done
clear
B)
At H/2 metre from the ground
done
clear
C)
H/6 metre from the ground
done
clear
D)
At H/4 metre from the ground
done
clear
View Answer play_arrow
question_answer 39) A bullet of mass 10 g leaves a rifle at an initial velocity of 1000 m/s and strikes the earth at the same level with a velocity of 500 m/s. The work in overcoming the resistance of air will be:
A)
500 J
done
clear
B)
5000 J
done
clear
C)
3750 J
done
clear
D)
475 J
done
clear
View Answer play_arrow
question_answer 40) A hole is made at the bottom of the tank filled with water (density 1000 kg/m3). If the total pressure at the bottom of the tank is 3 atmosphere (1 atmosphere=\[{{E}_{q}}\]), then the velocity of efflux is:
A)
\[{{E}_{a}}=4{{E}_{q}}\]
done
clear
B)
\[{{E}_{q}}=2{{E}_{a}}\]
done
clear
C)
\[{{E}_{a}}=2{{E}_{q}}\]
done
clear
D)
\[{{E}_{q}}=3{{E}_{a}}\]
done
clear
View Answer play_arrow
question_answer 41) Potential energy of a satellite having mass m and rotating at a height of \[6.4\times {{10}^{6}}m\] from the earth centre is :
A)
-0.2\[I-D,II-C,III-A,IV-B\],
done
clear
B)
-2\[I-C,II-D,III-A,IV-B\],
done
clear
C)
-0.5\[I-B,II-D,III-A,IV-C\]
done
clear
D)
-\[I-A,II-B,III-C,IV-D\],
done
clear
View Answer play_arrow
question_answer 42) During the adiabatic expansion of two moles of a gas the internal energy of a gas is found to decrease by 2, J. The work done during the process on gas will be equal to:
A)
-2 J
done
clear
B)
3 J
done
clear
C)
1 J
done
clear
D)
2 J
done
clear
View Answer play_arrow
question_answer 43) The real coefficient of volume expansion of glycerine is 0.000597 per \[{}^\circ C\] and linear coefficient of expansion of glass is 0.000009 per \[{}^\circ C\]. Then, the apparent volume coefficient of expansion of glycerine is :
A)
0.000558 per \[{}^\circ C\]
done
clear
B)
0.00057 per \[{}^\circ C\]
done
clear
C)
0.00027 per \[{}^\circ C\]
done
clear
D)
0.00066 per \[{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 44) An equilateral prism is made of a material of refractive index \[\frac{4}{5}D\].The angle of minimum deviation for the prism is:
A)
\[90{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 45) In an electron microscope the accelerating voltage is increased from 20 kV to 80 kV, the resolving power of the microscope will become:
A)
2 R
done
clear
B)
\[\frac{D}{2}\]
done
clear
C)
4 R
done
clear
D)
3 R
done
clear
View Answer play_arrow
question_answer 46) A solenoid is 1.5 m long and its inner diameter is 4.0 cm. It has 3 layers of windings of 1000 turns each and carries a current of 2.0 A. The magnetic flux for a cross-section of the solenoid is nearly:
A)
\[\frac{3D}{4}\]
done
clear
B)
\[\frac{5}{4}D\]
done
clear
C)
\[\frac{h}{\pi }\]
done
clear
D)
\[\frac{2h}{\pi }\]
done
clear
View Answer play_arrow
question_answer 47) Which one of the following is true about the p-type and n-type semiconductor?
A)
n-type semi-conductor have holes in majority
done
clear
B)
The concentration of electrons and holes are equal in both n-type semiconductors
done
clear
C)
n-type semiconductors have free electrons in majority
done
clear
D)
n-type semiconductor has excess negative charge
done
clear
View Answer play_arrow
question_answer 48) Which one of the following are used to express intensity of magnetic field in vacuum?
A)
Oersted
done
clear
B)
Tesla
done
clear
C)
Gauss
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 49)
Two cars A and B approach a stationary observer from opposite sides as shown in figure. Observer hears no beats. If the frequency of the horn of the car B is 504 Hz, the frequency of horn of car A will be :
A)
529.2 Hz
done
clear
B)
295.2 Hz
done
clear
C)
440.5 Hz
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 50) The surface of zone material is radiated in turn, by waves of \[\frac{3h}{2\pi }\]= 350 nm and 540 nm respectively. The ratio of the stopping potential in the two cases is 2 : 1. The work function of the material is :
A)
4.20 eV
done
clear
B)
0.15 eV
done
clear
C)
2.10 eV
done
clear
D)
1.05 eV
done
clear
View Answer play_arrow
question_answer 51) Assertion: Cyclotron does not accelerate electron. Reason: Mass of the electron is very small.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 52) Assertion: The average speed of an object may be equal to arithmetic mean of individual speeds. Reason: Average speeds is equal to total distance travelled per total lime Liken.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 53) Assertion: A spaceship while entering the earths atmosphere is likely to catch fire. Reason: The temperature of upper atmosphere is very high.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 54) Assertion: A balloon filled with hydrogen will fall with acceleration \[2\pi h\]of the moon. Reason: Moon has no atmosphere.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 55) Assertion: For gas atom the number of degrees of freedom is 3. Reason: -\[[M{{L}^{2}}{{T}^{-2}}]\]
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 56) Assertion: A turning fork is m resonance with a closed pipe. But the same tuning fork cannot be in resonance with an open pipe of the same length. Reason: The same tuning fork will not be in resonance with open pipe of same length due to end correction of pipe.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 57) Assertion: The refractive index of diamond is \[[M{{L}^{-1}}{{T}^{-1}}]\] and that of liquid is \[[{{M}^{-1}}{{L}^{3}}{{T}^{-2}}]\]. If tile light travels from diamond to the liquid, it will be totally reflected when the angle of incidence is 30°. Reason: \[2\times {{10}^{-4}}J\], where n is the refractive index of diamond with respect to liquid.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 58) Assertion: The setting sun appears to be red. Reason: Scattering of light is directly proportional to the wavelength.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 59) Assertion: If the speed of charged particle increases both the mass as well as charge increases. Reason: If \[{{m}_{o}}\] be rest mass and m the mass at velocity v then \[2\times {{10}^{-8}}N{{m}^{-1}}\] where c = speed of light.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 60) Assertion: Mass of moving photon varies inversely as the wavelength. Reason: Energy of the particle = mass \[\times \] (speed of light)2.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 61) Ethyl alcohol reacts with chlorine to produce:
A)
\[C{{H}_{3}}C{{H}_{2}}Cl\]
done
clear
B)
\[C{{H}_{3}}ClC{{H}_{2}}OH\]
done
clear
C)
\[CHC{{l}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[CC{{l}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 62) Which of the following statement is false for the reaction \[{{H}_{2}}+B{{r}_{2}}\xrightarrow{{}}2HBr\]? The rate law is \[\frac{dx}{dt}=k[{{H}_{2}}]{{[B{{r}_{2}}]}^{1/2}}\].
A)
Order of reaction is 1.5
done
clear
B)
Molecularity of the reaction is 2
done
clear
C)
By increasing the concentration of \[B{{r}_{2}}\] four times the; rate of reaction is doubled
done
clear
D)
All the above are correct
done
clear
View Answer play_arrow
question_answer 63) For reducing one mole of \[C{{r}_{2}}O_{7}^{2-}\] to \[C{{r}^{3+}}\] the charge required is:
A)
\[3\times 96500\text{ }C\]
done
clear
B)
\[6\times 96500\text{ }C\]
done
clear
C)
\[0.3\text{ }F\]
done
clear
D)
\[0.6\text{ }F\]
done
clear
View Answer play_arrow
question_answer 64) Which of the -following compound is formed when \[C{{H}_{2}}={{(C{{H}_{2}})}_{2}}COOH\] react with \[HBr\]?
A)
\[C{{H}_{3}}CHC{{H}_{2}}C{{H}_{2}}BrCOOH\]
done
clear
B)
\[C{{H}_{3}}CHBrC{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{2}}BrC{{H}_{2}}{{(C{{H}_{2}})}_{2}}COOH\]
done
clear
D)
\[C{{H}_{3}}CHC{{H}_{2}}BrC{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 65) At 298 K equal volumes of \[S{{O}_{2}}\], \[C{{H}_{4}}\] and \[{{O}_{2}}\] are mixed in empty container. The total pressure exerted is 2.1 atm. The partial pressure of \[C{{H}_{4}}\] in mixture is:
A)
0.6 atm
done
clear
B)
1.2 atm
done
clear
C)
2.4 atm
done
clear
D)
3.6 atm
done
clear
View Answer play_arrow
question_answer 66) \[A+2B\rightleftharpoons 2C+D\] initial concentration of B was 1.5 times that of A, but the equilibrium concentration of A and B are found to be equal. The equilibrium constant for the reaction is:
A)
4
done
clear
B)
8
done
clear
C)
12
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 67) Which of the following is greatest paramagnetic?
A)
\[C{{u}^{+}}\]
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[F{{e}^{3+}}\]
done
clear
D)
\[C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 68) Deuterium nucleus contains:
A)
1 proton 1 electron
done
clear
B)
1 proton 1 neutron
done
clear
C)
2 proton 1 electron
done
clear
D)
1 proton 2 electron
done
clear
View Answer play_arrow
question_answer 69) The correct order of hydration energy of alkali metal is:
A)
\[L{{i}^{+}}>N{{a}^{+}}>{{K}^{+}}>R{{b}^{+}}\]
done
clear
B)
\[R{{b}^{+}}>{{K}^{+}}>N{{a}^{+}}>L{{i}^{+}}\]
done
clear
C)
\[N{{a}^{+}}>{{K}^{+}}>L{{i}^{+}}>R{{b}^{+}}\]
done
clear
D)
\[{{K}^{+}}>R{{b}^{+}}>N{{a}^{+}}>L{{i}^{+}}\]
done
clear
View Answer play_arrow
question_answer 70) The EAN of Zn in \[{{[Zn{{(OH)}_{4}}]}^{2-}}\] complex is:
A)
16
done
clear
B)
26
done
clear
C)
36
done
clear
D)
46
done
clear
View Answer play_arrow
question_answer 71) During isothermal expansion of one mole of an Ideal gas from 10 atm to 1 atm at 273 K, the work done is [gas constant = 2 ]:
A)
\[-895.8\text{ }cal\]
done
clear
B)
\[-1172.6\text{ }cal\]
done
clear
C)
\[-1381.8\text{ }cal~~~~\]
done
clear
D)
\[-1499.6\text{ }cal\]
done
clear
View Answer play_arrow
question_answer 72) Which of the following is the atomic number of metal?
A)
32
done
clear
B)
34
done
clear
C)
36
done
clear
D)
38
done
clear
View Answer play_arrow
question_answer 73) The Mohrs salt is shown by:
A)
\[FeS{{O}_{4}}{{(N{{H}_{4}})}_{2}}S{{O}_{4}}.6{{H}_{2}}O\]
done
clear
B)
\[FeS{{O}_{4}}{{(N{{H}_{3}})}_{2}}S{{O}_{4}}.6{{H}_{2}}O\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}A{{l}_{2}}{{(S{{O}_{4}})}_{3}}.24{{H}_{2}}O\]
done
clear
D)
\[FeS{{O}_{2}}{{(N{{H}_{2}})}_{4}}S{{O}_{4}}.6{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 74) Mac Arthur process is used for the extraction of:
A)
Au
done
clear
B)
Ag
done
clear
C)
Cu
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 75) \[p{{K}_{a}}\] value of four acids are given below. The strongest acid is:
A)
4.0
done
clear
B)
3.5
done
clear
C)
2.5
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 76) The oxidation number of sulphur in \[{{H}_{2}}{{S}_{2}}{{O}_{7}}\] is:
A)
\[+2\]
done
clear
B)
\[+6\]
done
clear
C)
\[+4\]
done
clear
D)
\[+8\]
done
clear
View Answer play_arrow
question_answer 77) One mole of an Ideal gas for which \[{{C}_{v}}=3/2R\] is heated reversibly at a constant pressure of 1 atm from \[{{25}^{o}}C\] to \[{{100}^{o}}C\]. The \[\Delta H\] is:
A)
3.775 cal
done
clear
B)
37.256 cal
done
clear
C)
372.56 cal
done
clear
D)
3725.6 cal
done
clear
View Answer play_arrow
question_answer 78) Vant Hoff factor is:
A)
more than one in case of association
done
clear
B)
less than one in case of dissociation
done
clear
C)
equal to \[\frac{normal\,molecular\,mass}{observed\,molecular\,mass}\]
done
clear
D)
equal to \[\frac{observed\,molecular\,mass}{normal\,molecular\,mass}\]
done
clear
View Answer play_arrow
question_answer 79) Solid \[C{{O}_{2}}\] is known as dry ice:
A)
it melts at \[{{0}^{o}}C\]
done
clear
B)
its BP is more than \[{{199}^{o}}C\]
done
clear
C)
it directly change to vapours lowering the temperature to \[-{{78}^{o}}C\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 80) Which of the following statement is not correct regarding hydrogen atom?
A)
It resembles with halogens in some properties
done
clear
B)
It resembles with alkali metals in some properties,
done
clear
C)
It cannot be placed in first group of periodic table
done
clear
D)
It is the lightest element
done
clear
View Answer play_arrow
question_answer 81)
A)
p-chloro nitrobenzene
done
clear
B)
m-chloro nitrobenzene
done
clear
C)
o-chloro nitrobenzene
done
clear
D)
o-p-dichloro nitrobenzene
done
clear
View Answer play_arrow
question_answer 82) Glucose gives silver mirror with Tollens reagent; This shows the presence or:
A)
\[-COOH\]
done
clear
B)
\[-OH\]
done
clear
C)
\[-COH\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 83) Deficiency of vitamin-D causes:
A)
night blindness
done
clear
B)
rickets
done
clear
C)
scurvy
done
clear
D)
loss of appetite
done
clear
View Answer play_arrow
question_answer 84) Nitrolim is:
A)
\[Ca{{C}_{2}}\] and graphite
done
clear
B)
\[CaC{{N}_{2}}\] and graphite
done
clear
C)
\[Ca{{(CN)}_{2}}\] and graphite
done
clear
D)
\[CaC{{N}_{2}}+{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 85) On mixing 3 g of non-volatile solute in 200 mL of water its boiling point \[({{100}^{o}}C)\] becomes \[{{100.52}^{o}}C\]. If \[{{k}_{b}}\] for water is 0.6 K.kg/mol then molecular weight of solute is:
A)
\[10.5g\,mo{{l}^{-1}}\]
done
clear
B)
\[12.6\text{ }g\,mo{{l}^{-1}}\]
done
clear
C)
\[15.7\text{ }g\,mo{{l}^{-1}}\]
done
clear
D)
\[17.3\text{ }g\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 86) The outermost configuration of most electronegative element is:
A)
\[n{{s}^{2}}n{{p}^{5}}\]
done
clear
B)
\[n{{s}^{2}}n{{p}^{6}}\]
done
clear
C)
\[n{{s}^{2}}n{{p}^{4}}\]
done
clear
D)
\[n{{s}^{2}}n{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 87) Picric acid is:
A)
trinitrophenol
done
clear
B)
trinitrotoluene
done
clear
C)
trinitrobenzene
done
clear
D)
tribromobenzene
done
clear
View Answer play_arrow
question_answer 88)
A)
Streckers reaction
done
clear
B)
Sandmeyers reaction
done
clear
C)
Wolff-Kishner reaction
done
clear
D)
Stephens reaction
done
clear
View Answer play_arrow
question_answer 89) Ostwalds dilution law is applicable on:
A)
strong electrolytes
done
clear
B)
weak electrolytes
done
clear
C)
both strong and weak electrolytes
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 90) Which equation shows correct form of Berthelot equation?
A)
\[\left( P+\frac{a}{T{{(V+C)}^{2}}} \right)(V-b)=RT\]
done
clear
B)
\[\left( P+\frac{a}{T{{(V-C)}^{2}}} \right)(V-b)=RT\]
done
clear
C)
\[\left( P+\frac{a}{T{{V}^{2}}} \right)(V-b)=RT\]
done
clear
D)
\[\left( P+\frac{a}{T{{V}^{2}}} \right)(V+b)=RT\]
done
clear
View Answer play_arrow
question_answer 91) Lucas reagent is:
A)
\[arihy.\,AlC{{l}_{3}}+conc.HCl\]
done
clear
B)
\[anhy.AlC{{l}_{3}}+\text{ }cone.\text{ }HN{{O}_{3}}\]
done
clear
C)
\[anhy.ZnC{{l}_{2}}\]
done
clear
D)
\[anhy.ZnC{{l}_{2}}cone.HCl\]
done
clear
View Answer play_arrow
question_answer 92) Enthalpy of neutralization of \[C{{H}_{3}}COOH\]by. \[NaOH\] is \[-50.6kJ/mol\] and the heat of neutralisation of a strong acid with \[NaOH\] is \[-55.9kJ/mol\]. The value of \[\Delta H\] for the ionisation of \[NaOH\] is :
A)
\[3.5kJ/mol\]
done
clear
B)
\[4.6kJ/mol\]
done
clear
C)
\[5.3kJ/mol\]
done
clear
D)
\[6.4kJ/mol\]
done
clear
View Answer play_arrow
question_answer 93) Producer gas is mixture of:
A)
\[CO+{{N}_{2}}\]
done
clear
B)
\[CO+{{H}_{2}}\]
done
clear
C)
\[CO+{{H}_{2}}+{{O}_{2}}\]
done
clear
D)
\[CO+{{H}_{2}}+{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 94) In the following chemical reaction: \[AgO+{{H}_{2}}O+2{{e}^{-}}\xrightarrow{{}}2Ag+2O{{H}^{-}}\]
A)
hydrogen is reduced
done
clear
B)
electrons are reduced
done
clear
C)
water is oxidized
done
clear
D)
silver is oxidised
done
clear
View Answer play_arrow
question_answer 95) The molecular formula of plaster of Paris is:
A)
\[2CaS{{O}_{4}}.{{H}_{2}}O\]
done
clear
B)
\[CaS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
C)
\[CaS{{O}_{4}}.3{{H}_{2}}O\]
done
clear
D)
\[2CaS{{O}_{4}}.1/2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 96) Which of the following is the sweetest sugar?
A)
Glucose
done
clear
B)
Fructose
done
clear
C)
Sucrose
done
clear
D)
Maltose
done
clear
View Answer play_arrow
question_answer 97) Aldol condensation does not take place in:
A)
\[HCHO~\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 98) Which of the following has maximum ionisation potential?
A)
\[Al\]
done
clear
B)
\[P\]
done
clear
C)
\[Si\]
done
clear
D)
\[Mg\]
done
clear
View Answer play_arrow
question_answer 99) Acetic acid on heating with \[{{P}_{2}}{{O}_{5}}\] produce:
A)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
\[{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 100) Which pair have same percentage of carbon?
A)
\[C{{H}_{3}}COOH\]and \[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]
done
clear
B)
\[C{{H}_{3}}COOH\]and \[{{C}_{12}}{{H}_{22}}{{O}_{11}}\]
done
clear
C)
\[C{{H}_{3}}COOH\] and \[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\] and \[{{C}_{12}}{{H}_{22}}{{O}_{11}}\]
done
clear
View Answer play_arrow
question_answer 101) Bohrs theory is not applicable to:
A)
\[H\]
done
clear
B)
\[H{{e}^{+}}\]
done
clear
C)
\[L{{i}^{2+}}\]
done
clear
D)
\[{{H}^{+}}\]
done
clear
View Answer play_arrow
question_answer 102) The de-Broglie wavelength of the electron in the ground state of hydrogen atom is: \[[KE=13.6eV];\,eV=1.602\times {{10}^{-19}}J\]
A)
33.28 nm
done
clear
B)
3.328 nm
done
clear
C)
0.3328 nm
done
clear
D)
0.0332nm
done
clear
View Answer play_arrow
question_answer 103) 0.4 moles of \[HCl\] and 0.2 moles of \[CaC{{l}_{2}}\] were dissolved in water to have 500 mL of solution, the molarity of \[C{{l}^{-}}\] ion is:
A)
0.8 M
done
clear
B)
1.6 M
done
clear
C)
1.2M
done
clear
D)
10.0 M
done
clear
View Answer play_arrow
question_answer 104) Milk is colloid in which:
A)
liquid is dispersed in liquid
done
clear
B)
gas is dispersed in liquid
done
clear
C)
sugar is dispersed in water
done
clear
D)
solid is dispersed in liquid
done
clear
View Answer play_arrow
question_answer 105) Hypo on treatment with iodine produce:
A)
\[N{{a}_{2}}S\]
done
clear
B)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
C)
\[N{{a}_{2}}S{{O}_{3}}\]
done
clear
D)
\[N{{a}_{2}}{{S}_{4}}{{O}_{6}}\]
done
clear
View Answer play_arrow
question_answer 106) When 8.3 g copper sulphate reacts with excess of potassium iodide then the amount of iodine liberated is:
A)
42.3 g
done
clear
B)
24.3 g
done
clear
C)
4.23 g
done
clear
D)
2.43 g
done
clear
View Answer play_arrow
question_answer 107) Glucose reacts with phenyl-hydrazine to produce osazone. Number of molecules of phenyl-hydrazine take part is:
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 108) German silver is an alloy of:
A)
\[Fe,Cr,Ni\]
done
clear
B)
\[Ag,Cu,Au\]
done
clear
C)
\[Cu,Zn,Ni\]
done
clear
D)
\[Cu,Zn,Sn\]
done
clear
View Answer play_arrow
question_answer 109) Which of the following molecule contains one lone pair of electron on the central atom?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 110) Amylose is a polymer of:
A)
\[\beta -D\] glucose
done
clear
B)
\[\alpha -D\] glucopyranose
done
clear
C)
fructose
done
clear
D)
\[\beta -\] fructose
done
clear
View Answer play_arrow
question_answer 111) Assertion: Sucrose undergo mutarotation. Reason: Sucrose is a disaccharide.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 112) Assertion: Resorcinol turns \[FeC{{l}_{3}}\] solution purple. Reason: Resorcinol have phenolic group.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 113) Assertion: Copper reacts with \[HCl\] and liberates hydrogen. Reason: Hydrogen is present above \[Cu\] in the reactivity series.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 114) Assertion: \[N{{H}_{3}}\] absorbs more readily over activated charcoal than \[C{{O}_{2}}\]. Reason: \[N{{H}_{3}}\] is non-polar.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 115) Assertion: Sky appears blue colour. Reason: Colloidal particles of dust scatter blue light.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 116) Assertion: Acetylene on treatment with alkaline \[KMn{{O}_{4}}\] produce acetaldehyde. Reason: Alkaline \[KMn{{O}_{4}}\] is a reducing agent.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 117) Assertion: Entropy of ice is less than water. Reason: Ice have cage like structure.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 118) Assertion: Use of pressure cooker reduces cooking time. Reason: At higher pressure cooking occurs faster.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 119) Assertion: Lime water becomes turbid on passing \[C{{O}_{2}}\] but becomes clear on passing more \[C{{O}_{2}}\]. Reason: Lime water is calcium hydroxide, \[Ca{{(OH)}_{2}}\].
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 120) Assertion: The atoms of different elements having same mass number but different atomic number are known as isobars. Reason: The sum of protons and neutrons in isobars is always different.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 121) Rough E.R. differs from smooth E.R. due to the presence of:
A)
DNA
done
clear
B)
nucleus
done
clear
C)
ribosome
done
clear
D)
enzyme
done
clear
View Answer play_arrow
question_answer 122) HIV has a protein coat and genetic material:
A)
ss RNA
done
clear
B)
ds RNA
done
clear
C)
ss DNA
done
clear
D)
ds DNA
done
clear
View Answer play_arrow
question_answer 123) Apoenzyme is:
A)
protein
done
clear
B)
lipid
done
clear
C)
sugar
done
clear
D)
vitamin
done
clear
View Answer play_arrow
question_answer 124) The basic unit of classification is:
A)
genus
done
clear
B)
species
done
clear
C)
variety
done
clear
D)
subspecies
done
clear
View Answer play_arrow
question_answer 125) The role of bacteria in carbon cycle is:
A)
photosynthesis
done
clear
B)
chemosynthesis
done
clear
C)
decomposition of organic compounds
done
clear
D)
evolution of
done
clear
View Answer play_arrow
question_answer 126) 13 celled male gametophyte of Selaginella has:
A)
12 cells of antheridium +1 prothallial cell
done
clear
B)
10 cell of antheridium + 3 prothallial cell
done
clear
C)
9 cell of antheridium + 4 prothallial cell
done
clear
D)
8 cells of antheridium + 6 prothallial cell
done
clear
View Answer play_arrow
question_answer 127) The edible part of cauliflower:
A)
inflorescence
done
clear
B)
leaf
done
clear
C)
flower
done
clear
D)
stem
done
clear
View Answer play_arrow
question_answer 128) 10% law of energy transfer was given by:
A)
Lindmann
done
clear
B)
Tansley
done
clear
C)
Stanely
done
clear
D)
Darwin
done
clear
View Answer play_arrow
question_answer 129) Cambium of root is an example of:
A)
Apical meristem
done
clear
B)
Intercalary meristem
done
clear
C)
Primary meristem
done
clear
D)
Secondary meristem
done
clear
View Answer play_arrow
question_answer 130) Growth of pollen tube towards embryo is:
A)
geotropism
done
clear
B)
chemotaxis
done
clear
C)
phototaxis
done
clear
D)
thigmotaxis
done
clear
View Answer play_arrow
question_answer 131) Which of the following statement is true?
A)
Spores are gametes
done
clear
B)
Spores and gametes are diploid
done
clear
C)
Gametes are always haploid
done
clear
D)
Spores are always diploid
done
clear
View Answer play_arrow
question_answer 132) Which of the following is found in algal zone of Cycas coralloid roots?
A)
Blue-green algae
done
clear
B)
Red algae
done
clear
C)
Diatoms
done
clear
D)
Brown algae
done
clear
View Answer play_arrow
question_answer 133) Acid rain is due to pollution of:
A)
dust
done
clear
B)
pesticides
done
clear
C)
\[S{{O}_{2}}\] and \[N{{O}_{2}}\]
done
clear
D)
carbon particle
done
clear
View Answer play_arrow
question_answer 134) The egg case in female cockroach is formed by secretion of:
A)
collaterial gland
done
clear
B)
mushroom gland
done
clear
C)
conglobate gland
done
clear
D)
prothoraic gland
done
clear
View Answer play_arrow
question_answer 135) Haploid cultures can be obtained by culturing
A)
pollen grains
done
clear
B)
embryo
done
clear
C)
shoot apex
done
clear
D)
root apex
done
clear
View Answer play_arrow
question_answer 136) Most reduced form of stem is found in:
A)
bulb
done
clear
B)
rhizome
done
clear
C)
tree
done
clear
D)
stem
done
clear
View Answer play_arrow
question_answer 137) Chromosomes with equal arms are called:
A)
metacentric
done
clear
B)
telocentric
done
clear
C)
acentric
done
clear
D)
polycentric
done
clear
View Answer play_arrow
question_answer 138) Colchicine prevents the mitosis of cell at which of following stage?
A)
Anaphase
done
clear
B)
Metaphase
done
clear
C)
Prophase
done
clear
D)
Interphase
done
clear
View Answer play_arrow
question_answer 139) In Opuntiu spines are modification of:
A)
stem
done
clear
B)
root
done
clear
C)
leaf
done
clear
D)
flower
done
clear
View Answer play_arrow
question_answer 140) Which of the following antibiotic discovered by Alexander Fleming?
A)
Streptomycin
done
clear
B)
Tetracycline
done
clear
C)
Penicillin
done
clear
D)
Terramycin
done
clear
View Answer play_arrow
question_answer 141) Glycolysis occur in:
A)
mitochondria
done
clear
B)
chloroplast
done
clear
C)
parenchyma
done
clear
D)
sieve tube cell
done
clear
View Answer play_arrow
question_answer 142) Which of the following maturity?
A)
Companion cell
done
clear
B)
Meristematic
done
clear
C)
Parenchyma
done
clear
D)
sieve tube cell
done
clear
View Answer play_arrow
question_answer 143) Food chain starts with:
A)
autotrophs
done
clear
B)
herbivores
done
clear
C)
carnivores
done
clear
D)
decomposers
done
clear
View Answer play_arrow
question_answer 144) The plants which can withstand narrow range of temperature tolerance, are called:
A)
stenothermal
done
clear
B)
eurythermal
done
clear
C)
mesothermal
done
clear
D)
monothermal
done
clear
View Answer play_arrow
question_answer 145) Which of the following is a fungus?
A)
Nostoc
done
clear
B)
E. coli
done
clear
C)
Yeast
done
clear
D)
Chara
done
clear
View Answer play_arrow
question_answer 146) Okazaki fragments form:
A)
leading strand
done
clear
B)
lagging strand
done
clear
C)
non sense strand
done
clear
D)
senseful strand
done
clear
View Answer play_arrow
question_answer 147) Power of regeneration of sponges is due to:
A)
theocytes
done
clear
B)
archaeocytes
done
clear
C)
amoebocytes
done
clear
D)
sclerocytes
done
clear
View Answer play_arrow
question_answer 148) The poisonous fluid present in nematocysts of Hydra is:
A)
toxin
done
clear
B)
venom
done
clear
C)
hematin
done
clear
D)
hypnotoxin
done
clear
View Answer play_arrow
question_answer 149) Electron microscope was invented by:
A)
Robert Hooke
done
clear
B)
Knoll and Ruska
done
clear
C)
Pasteur
done
clear
D)
Schwann and Schleiden
done
clear
View Answer play_arrow
question_answer 150) Flora and fauna in lake or ponds is:
A)
lentic biota
done
clear
B)
biotic biota
done
clear
C)
abiotic biota
done
clear
D)
field layer
done
clear
View Answer play_arrow
question_answer 151) Polygenic genes show:
A)
similar genotype
done
clear
B)
different phenotype
done
clear
C)
different karyotype
done
clear
D)
different genotype
done
clear
View Answer play_arrow
question_answer 152) The enzyme responsible for the reduction of molecular nitrogen to the level of ammonia in the leguminous root nodule:
A)
nitrogenase
done
clear
B)
nitrate reductase
done
clear
C)
nitrite reductase
done
clear
D)
amminase
done
clear
View Answer play_arrow
question_answer 153) Malignant tertain malaria is caused by:
A)
P. vivax
done
clear
B)
P. malariae
done
clear
C)
P. ovale
done
clear
D)
P. falciparum
done
clear
View Answer play_arrow
question_answer 154) Life cycle of Taenia is:
A)
monogenetic
done
clear
B)
digenetic
done
clear
C)
polygenetic
done
clear
D)
hexogenetic
done
clear
View Answer play_arrow
question_answer 155) Pigment haemocyanin is found in:
A)
Chordata
done
clear
B)
Annelida
done
clear
C)
Mollusca
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 156) Antedon belong to which of the following class?
A)
Asteroidea
done
clear
B)
Ophiuroidea
done
clear
C)
Crinoidea
done
clear
D)
Echinoidea
done
clear
View Answer play_arrow
question_answer 157) Scales in Chondrichthyes are:
A)
placoid
done
clear
B)
canoid
done
clear
C)
cycloid
done
clear
D)
sysamoid
done
clear
View Answer play_arrow
question_answer 158) Which of the following snake is not poisonous?
A)
Naja-naja
done
clear
B)
Python
done
clear
C)
Hydrophis
done
clear
D)
Bungarus
done
clear
View Answer play_arrow
question_answer 159) Birds are:
A)
cold blooded
done
clear
B)
homeothermal
done
clear
C)
poikilothermal
done
clear
D)
homeopoiesis
done
clear
View Answer play_arrow
question_answer 160) Cranium of human contains:
A)
12 bones
done
clear
B)
8 bones
done
clear
C)
14 bones
done
clear
D)
20 bones
done
clear
View Answer play_arrow
question_answer 161) Which of the following sub-stances is at it lowest level in fish food?
A)
Acting
done
clear
B)
Myosin
done
clear
C)
Cholesterol
done
clear
D)
Tissue fluid
done
clear
View Answer play_arrow
question_answer 162) Reabsorption in tubules of nephrons occurs by:
A)
Osmosis
done
clear
B)
diffusion
done
clear
C)
Alteration
done
clear
D)
active transport
done
clear
View Answer play_arrow
question_answer 163) Which disease has XXY chromosome constitution?
A)
Downs syndrome
done
clear
B)
Turners syndrome
done
clear
C)
Klinefelters syndrome
done
clear
D)
Okazaki syndrome
done
clear
View Answer play_arrow
question_answer 164) Cell wall is absent in:
A)
Amoeba
done
clear
B)
Chara
done
clear
C)
Yeast
done
clear
D)
E. coli
done
clear
View Answer play_arrow
question_answer 165) Gamma globulin are synthesized inside:
A)
liver
done
clear
B)
kidney
done
clear
C)
bone marrow
done
clear
D)
lymph and lymphoid tissue
done
clear
View Answer play_arrow
question_answer 166) Double membrane structure of cell are:
A)
Nucleus
done
clear
B)
chloroplast
done
clear
C)
mitochondria
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 167) Binomial nomenclature was introduced by:
A)
Linnaeus
done
clear
B)
Darwin
done
clear
C)
Bentham and Hooker
done
clear
D)
Aristotle
done
clear
View Answer play_arrow
question_answer 168) The name of vitamin C is:
A)
ascorbic acid
done
clear
B)
glutamic acid
done
clear
C)
aspartic acid
done
clear
D)
enolic acid
done
clear
View Answer play_arrow
question_answer 169) Hydrolytic enzymes are found in:
A)
peroxisomes
done
clear
B)
lysosomes
done
clear
C)
lepdosomes
done
clear
D)
losmasomes
done
clear
View Answer play_arrow
question_answer 170) How may ovaries are found in birds?
A)
One ovary
done
clear
B)
Two ovaries
done
clear
C)
Three ovaries
done
clear
D)
Many ovaries
done
clear
View Answer play_arrow
question_answer 171) Assertion: In collateral vascular bundles phloem is situated towards inner side. Reason: In monocot stem, cambium is present.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 172) Assertion: Ginkgo biloga is living fossil. Reason: Organism which have persisted and remain unchanged for the past several million years while their relatives disappeared.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 173) Assertion: Saline water is not given to patients of hypertension. Reason: Saline water can cause vomiting and may drop blood pressure suddenly causing cardiac arrest.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 174) Assertion: Histones are basic proteins of major importance in packaging of eukaryotic DNA, DNA and histones comprise chromatin forming the bulk of eukaryotic chromosomes. Reason: Histones are five major types \[{{H}_{1}},\,{{H}_{2}}A,\,\,{{H}_{2}}B,\,\,{{H}_{3}}\] and \[{{H}_{4}}\].
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 175) Assertion: Bacteria have three basic shapes, i.e., round, rod, spiral. Reason: Cocci and bacilli may form clusters or chain of a definite length,
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 176) Assertion: Tongue is a gustatoreceptor. Reason: Receptors for gustatory sensations are located in taste buds.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 177) Assertion: Rabies, an infection of mammals which involve central nervous system, which may result in paralysis and finally death. Reason: This is caused by neurotropic bacteria in saliva of rabies animal.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 178) Assertion: Phenylketonuria is recessive hereditary disease caused by bodys failure to oxidize an amino acid phenylalanine to tryosine because of a defective enzyme. Reason: It results the presence of phenylalanine acid in urine.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 179) Assertion: Blood pressure is arterial blood pressure. Reason: Blood pressure is measured by sphygmomanometer.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 180) Assertion: Aflatoxins are produced by Aspergillus flavus. Reason: These toxins are useful to mankind.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 181) Williams cup is related to:
A)
basketball
done
clear
B)
table tennis
done
clear
C)
volley ball
done
clear
D)
foot ball
done
clear
View Answer play_arrow
question_answer 182) Sun city is in:
A)
USA
done
clear
B)
South Africa
done
clear
C)
France
done
clear
D)
Denmark
done
clear
View Answer play_arrow
question_answer 183) Full form of H.T.T.P. is:
A)
Hyper Terminal Transformation
done
clear
B)
Hyper Text Transfer Protocol
done
clear
C)
High Technology Test Principles
done
clear
D)
Hyper Taxt Training Programme
done
clear
View Answer play_arrow
question_answer 184) Tallest tower in the world is:
A)
C.N. Tower
done
clear
B)
Kutub Minar
done
clear
C)
Angel
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 185) The Satanic Verses a controversial book is written by:
A)
Gyani Jail Singh
done
clear
B)
Khushwant Singh
done
clear
C)
Kuldip Nayyar
done
clear
D)
Salman Rushdie
done
clear
View Answer play_arrow
question_answer 186) The contribution of Sarkaria commission was related between:
A)
state and centre
done
clear
B)
centre and union territories
done
clear
C)
one state to other state
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 187) World Tourism day was declared on:
A)
1st October
done
clear
B)
11th February
done
clear
C)
27th September
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 188) Weight of blood in the body is:
A)
about 7 litres in normal body or 7% of the total body weight
done
clear
B)
about 5 litres in normal body or 5% of the total body weight
done
clear
C)
about 10 litres in normal body or 10% of the body weight
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 189) Which one of first Indian missile (earth to earth) was tested successfully from Shri Hari Kota ?
A)
Prithvi
done
clear
B)
Nag
done
clear
C)
Agni
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 190) "Divine? comedy was written by:
A)
Goethe
done
clear
B)
Milton
done
clear
C)
Dante
done
clear
D)
Shakespears
done
clear
View Answer play_arrow
question_answer 191) Which one of the least poulated state in India?
A)
Nagaland
done
clear
B)
Himachal Pradesh
done
clear
C)
Orissa
done
clear
D)
Sikkim
done
clear
View Answer play_arrow
question_answer 192) Shakti Sthal is the name given to:
A)
The factory where Indias newly designed battle tanks are being manufactured
done
clear
B)
The samadhi of Indira Gandhi
done
clear
C)
The nuclear reactor at Kalpakam at Chennai
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 193) Kushi Nagar, the famous Buddhist pilgrimage centre in the state of:
A)
U.P.
done
clear
B)
M.P.
done
clear
C)
Bihar
done
clear
D)
Orissa
done
clear
View Answer play_arrow
question_answer 194) Abhigyan Shakuntalam was written by:
A)
Surdas
done
clear
B)
Tulsidas
done
clear
C)
R.N. Tagore
done
clear
D)
Kalidas
done
clear
View Answer play_arrow
question_answer 195) Nasik is situated on the bank of:
A)
Narmada
done
clear
B)
Krishna
done
clear
C)
Kauvery
done
clear
D)
Godavary
done
clear
View Answer play_arrow
question_answer 196) Which city is known as Pink city?
A)
Jaipur
done
clear
B)
Paris
done
clear
C)
New York
done
clear
D)
London
done
clear
View Answer play_arrow
question_answer 197) Grand Trunk road was built by:
A)
SherShah Suri
done
clear
B)
Shaha Jahan
done
clear
C)
Lord Bentick
done
clear
D)
Lord Mount Battan
done
clear
View Answer play_arrow
question_answer 198) Who was appointed as the first Indian Governor General of India?
A)
C. Raj Gopalachari
done
clear
B)
Radha Krishana
done
clear
C)
Y.C. Grace
done
clear
D)
V.V. Giri
done
clear
View Answer play_arrow
question_answer 199) Who was known as the ?Lady of the Lamp??
A)
Sarojini Naidu
done
clear
B)
Joan of Arc
done
clear
C)
Florence Nightinagale
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 200) Rial is the currency of:
A)
Afghanistan
done
clear
B)
Iran
done
clear
C)
Iraq
done
clear
D)
Jordan
done
clear
View Answer play_arrow
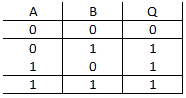 The truth table given for which of the following gates is correct?
The truth table given for which of the following gates is correct?

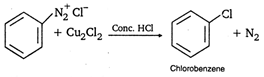 Above reaction is known as:
Above reaction is known as:
