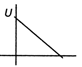-
question_answer1) A closely wound solenoid of 2000 turns and area of cross-section \[1.5\times {{10}^{-4}}{{m}^{2}}\] carries a current of 2.0 A. It is suspended through its centre and perpendicular to its length, allowing it to turn in a horizontal plane in a uniform magnetic field \[5\times {{10}^{-2}}{{m}^{2}}\] T, making an angle of\[30{}^\circ \] with the axis of the solenoid. The torque on the solenoid will he
A)
\[\text{3}\times \text{1}{{0}^{-\text{3}}}\text{ N}-\text{m}\]
done
clear
B)
\[1.5\times {{10}^{-3}}N-m\]
done
clear
C)
\[1.5\times {{10}^{-2}}N-m\]
done
clear
D)
\[3\times {{10}^{-2}}N-m\]
done
clear
View Answer play_arrow
-
question_answer2) A freshly prepared radioactive source of half-life 2 h emits radiation of intensity which is 64 times the permissible safe level. Calculate, the minimum time after which it would be possible to work safely with this source.
A)
12h
done
clear
B)
24 h
done
clear
C)
6 h
done
clear
D)
130 h
done
clear
View Answer play_arrow
-
question_answer3) A ball is droped from a high rise platform\[t=0\] starting from rest. After 6 s another ball is thrown downwards from the same platform with a speed u The two balls meet at \[t=18s\]s. What is the value of u?
A)
74 m/s
done
clear
B)
64 m/s
done
clear
C)
84 m/s
done
clear
D)
94 m/s
done
clear
View Answer play_arrow
-
question_answer4) The thermo emf E (in volts) of a certain thermocouple is found to vary with Q (in C) according to equation (\[E=20Q-\frac{{{Q}^{2}}}{20}\], where\[Q\]is temperature of the hot function, the cold function being kept at\[0{}^\circ C\]. Then, the neutral temperature of the thermocouple is
A)
\[300{}^\circ C\]
done
clear
B)
\[400{}^\circ C\]
done
clear
C)
\[100{}^\circ C\]
done
clear
D)
\[200{}^\circ C\]
done
clear
View Answer play_arrow
-
question_answer5) The maximum vertical distance through which a full dressed astronaut can jump on the earth is 0.5m. Estimate the maximum vertical distance through which he can jump on the moon, which has a mean density 2/3 rd that of the earth and radius one quarter that of the earth.
A)
1.5 m
done
clear
B)
3 m
done
clear
C)
6m
done
clear
D)
7.5 m
done
clear
View Answer play_arrow
-
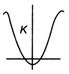
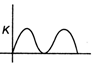
question_answer6)
As shown in figure a simple harmonic motion oscillator having identical four springs has time period 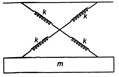
A)
\[T=2\pi \sqrt{\frac{m}{4k}}\]
done
clear
B)
\[T=2\pi \sqrt{\frac{m}{2k}}\]
done
clear
C)
\[T=2\pi \sqrt{\frac{m}{k}}\]
done
clear
D)
\[T=2\pi \sqrt{\frac{m}{8k}}\]
done
clear
View Answer play_arrow
-
question_answer7) If there were a reduction in gravitational effect, which of the following forces do you think would change in some respect?
A)
Magnetic force
done
clear
B)
Electrostatic force
done
clear
C)
Viscous force
done
clear
D)
Archimede's uplift
done
clear
View Answer play_arrow
-
question_answer8)
Two batteries of emf 4 V and 8 V with internal resistance \[1\Omega \]and \[2\Omega \] respectively are connected to an external resistance \[R=9\Omega \] as shown in figure. The current in circuit and the potential difference between P and Q respectively will be 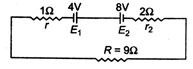
A)
\[\frac{1}{2}A,9V\]
done
clear
B)
\[\frac{1}{12}A,12V\]
done
clear
C)
\[\frac{1}{3}A,3V\]
done
clear
D)
\[\frac{1}{6}A,4V\]
done
clear
View Answer play_arrow
-
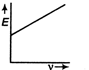
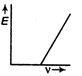
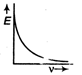
question_answer9) The correct graph respectively the relation between energy (E) of photoelectrons and frequency v of incident light is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer10) A body at a temperature of\[728{}^\circ C\]and has surface area\[5\text{ }c{{m}^{2}}\]radiates 300 J of energy each minute. The emissivity is (Given Boltzmann constant\[=\text{5}.\text{67}\times \text{1}{{\text{0}}^{-\text{8}}}\text{W}{{\text{m}}^{\text{2}}}{{\text{K}}^{\text{4}}})\]
A)
\[e=0.18\]
done
clear
B)
\[e=0.02\]
done
clear
C)
\[e=0.2\]
done
clear
D)
\[e=0.15\]
done
clear
View Answer play_arrow
-
question_answer11)
Considering normal incidence of ray, the equivalent refractive index of combination of two slabs shown in figure is | \[\mu =4/3\] |
| \[\mu =3/2\] |
A)
1.8
done
clear
B)
1.43
done
clear
C)
2
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer12) Three particles having charges in the ratio of 2.3:5 produce the same point on the photographic film in Thomson experiment Their masses are in the ratio of
A)
\[2:3:5\]
done
clear
B)
\[5:3:2\]
done
clear
C)
\[15:10:6\]
done
clear
D)
\[3:5:2\]
done
clear
View Answer play_arrow
-
question_answer13)
What will be ratio of speed in first two seconds to the speed in next 4s? 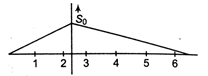
A)
\[\sqrt{2}:1\]
done
clear
B)
\[3:1\]
done
clear
C)
\[2:1\]
done
clear
D)
\[1:2\]
done
clear
View Answer play_arrow
-
question_answer14) A black body emits heat at the rate of 20 W When its temperature is\[727{}^\circ C\]. Another black body emits heat at the rate of 15W, when its temperature is\[227{}^\circ C\]. Compare the area of the surface of the two bodies, if the surrounding is at NTP.
A)
\[16:1\]
done
clear
B)
\[1:4\]
done
clear
C)
\[12:1\]
done
clear
D)
\[1:12\]
done
clear
View Answer play_arrow
-
question_answer15) The pressure on a square plate is measured by measuring the force on the plate and the length of the sides of the plate by using the formula\[P=\frac{F}{{{l}^{2}}}.\]If the maximum errors in the measurement of force and length are 4% and 2% respectively, then the maximum error in the measurement of pressure is
A)
1%
done
clear
B)
2%
done
clear
C)
8%
done
clear
D)
10%
done
clear
View Answer play_arrow
-
question_answer16) The transfer ratio \[\beta \]of a transistor is 50. The input resistance of the transistor, when used in the common emitter mode is\[1k\Omega .\]. The peak value of the collector alternating current for an input peak voltage of 0.01 V is
A)
\[0.25\mu A\]
done
clear
B)
\[0.01\mu A\]
done
clear
C)
\[500\mu A\]
done
clear
D)
\[100\mu A\]
done
clear
View Answer play_arrow
-
question_answer17) Four resistance \[\text{1}0\Omega ,\text{5}\Omega ,\text{7}\Omega \] and\[3\Omega \]. Are connected so that they form the side of a rectangle\[AB,BC,CD\]and DA respectively. Another resistance of \[10\Omega \] is connected across the diagonal AC. The equivalent resistance between A and B is
A)
\[2\Omega \]
done
clear
B)
\[5\Omega \]
done
clear
C)
\[7\Omega \]
done
clear
D)
\[10\Omega \]
done
clear
View Answer play_arrow
-
question_answer18) The velocity of a particle moving in the\[x-y\] plane is given by \[\frac{dx}{dt}=8\pi \sin 2\pi t\]and \[\frac{dy}{dt}=5\pi \cos 2\pi t\] where,\[t=0,\text{ }x=8\]and\[y=0,\]the path of the particle is
A)
a straight line
done
clear
B)
an ellipse
done
clear
C)
a circle
done
clear
D)
a parabola
done
clear
View Answer play_arrow
-
question_answer19) A rod of length L is hinged from one end. It is brought to a horizontal position and released. The angular velocity of the rod, when it is in vertical position is
A)
\[\sqrt{\frac{2g}{L}}\]
done
clear
B)
\[\sqrt{\frac{3g}{L}}\]
done
clear
C)
\[\sqrt{\frac{g}{2L}}\]
done
clear
D)
\[\sqrt{\frac{g}{L}}\]
done
clear
View Answer play_arrow
-
question_answer20) A weight w is suspended from the mid- point of a rope, whose ends are at the same level. In other to make the rope perfectly horizontal. the force applied to each of its ends must be
A)
less than w
done
clear
B)
equal to w
done
clear
C)
equal to 2 w
done
clear
D)
infinitely large
done
clear
View Answer play_arrow
-
question_answer21) A particle moves along a curve of unknown shape but magnitude offeree F is constant and always acts along tangent to the curve. Then,
A)
F may be conservative
done
clear
B)
F must be conservative
done
clear
C)
F may be non-conservative
done
clear
D)
F must be non-conservative
done
clear
View Answer play_arrow
-
question_answer22) A block has been placed on an inclined plane with the slope angle \[\theta ,\]block slide down the plane at constant speed. The coefficient of kinetic friction is equal to
A)
\[\sin \theta \]
done
clear
B)
\[\cos \theta \]
done
clear
C)
\[g\]
done
clear
D)
\[\tan \theta \]
done
clear
View Answer play_arrow
-
question_answer23) A charge q is located at the centre of a cube. The electric flux through any face is
A)
\[\frac{\pi q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
B)
\[\frac{q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
C)
\[\frac{2\pi q}{6(4\pi {{\varepsilon }_{0}})}\]
done
clear
D)
\[\frac{4\pi q}{\frac{1}{6}(4\pi {{\varepsilon }_{0}})}\]
done
clear
View Answer play_arrow
-
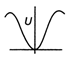
question_answer24) During SHM, a particle has displacement \[x\] form mean position. If acceleration. Kinetic energy and excess potential energy are represented by a K and U respectively, then choose the appropriate graph
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer25) The root mean square velocity of hydrogen molecule at 27°C is \[{{\upsilon }_{H}}\] and that of oxygen at 402°C is \[{{\upsilon }_{0}}\], then
A)
\[{{v}_{0}}>{{v}_{H}}\]
done
clear
B)
\[4{{v}_{0}}=9{{v}_{H}}\]
done
clear
C)
\[2{{v}_{0}}=3{{v}_{H}}\]
done
clear
D)
\[9{{v}_{0}}=134{{v}_{H}}\]
done
clear
View Answer play_arrow
-
question_answer26) A charged spherical conductor of radius a and charge g, is surrounded by another charged concentric sphere of radius\[b(b>a)\]. The potential difference between conductors is V. When, the spherical conductor of radius b is discharged completely, then the potential difference between conductor will be
A)
V
done
clear
B)
\[\frac{{{v}_{a}}}{b}\]
done
clear
C)
\[\frac{{{q}_{1}}}{4\pi {{\varepsilon }_{0}}a}-\frac{{{q}_{2}}}{4\pi {{\varepsilon }_{0}}b}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer27)
The current-voltage graph for a device is shown in figure. The resistance is negative in region. 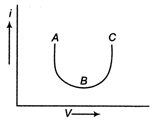
A)
AS
done
clear
B)
BC
done
clear
C)
ABC
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer28) Silver and copper voltmeters are connected in parallel with a battery of emf 12 V. In 30 min 1 g of silver and 1.8 g of copper are liberated. The energy supplied by the battery is
A)
720 J
done
clear
B)
2.41 J
done
clear
C)
24.12 J
done
clear
D)
\[4.34\times {{10}^{4}}\text{J}\]
done
clear
View Answer play_arrow
-
question_answer29) At a specific instant emission of radioactive compound is deflected in a magnetic field. The compound can emit
A)
electron
done
clear
B)
protons
done
clear
C)
\[H{{e}^{2+}}\]
done
clear
D)
neutrons
done
clear
View Answer play_arrow
-
question_answer30) A magnet is cut in three equal parts by cutting it perpendicular to its length. The time period of original magnet is \[{{T}_{0}}\] in a uniform magnetic field B. Then, the time period of each part in the same magnetic field is
A)
\[\frac{{{T}_{0}}}{2}\]
done
clear
B)
\[\frac{{{T}_{0}}}{3}\]
done
clear
C)
\[\frac{{{T}_{0}}}{4}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer31) A 50 Hz AC current of crest value 1 A flows through the primary of a transformer. If the mutual inductance between the primary and secondary be 0.5 H, the crest voltage induced in the secondary is
A)
75V
done
clear
B)
150V
done
clear
C)
100V
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer32) If the length and area of cross-section of a conductor are doubled, then its resistance will be
A)
unchanged
done
clear
B)
halved
done
clear
C)
doubled
done
clear
D)
quadrupled
done
clear
View Answer play_arrow
-
question_answer33) According to Wien's law
A)
\[{{\lambda }_{m}}T=\] constant
done
clear
B)
\[\frac{{{\lambda }_{m}}}{T}=\]constant
done
clear
C)
\[{{\lambda }_{m}}\sqrt{T}=\] constant
done
clear
D)
\[\frac{{{\lambda }_{m}}}{\sqrt{T}}=\]constant
done
clear
View Answer play_arrow
-
question_answer34) A source of light lies on the angle bisector of two plane mirrors inclined at angle \[\theta \]. The values of \[\theta \], so that the light reflected from one mirror does not reach the other mirror will be
A)
\[\theta \ge \text{12}0{}^\circ \]
done
clear
B)
\[\theta \ge \text{9}0{}^\circ \]
done
clear
C)
\[\theta \le \text{12}0{}^\circ \]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer35) A ruby laser produces radiations of wavelengths, 662.6nm in pulse whose duration are \[\text{1}{{0}^{-\text{9}}}\] s. If the laser produces 0.39 J of energy per pulse, how many photons are produced in each pulse?
A)
\[\text{1}.\text{3}\times \text{1}{{0}^{\text{9}}}\]
done
clear
B)
\[\text{1}.\text{3}\times \text{1}{{0}^{\text{18}}}\]
done
clear
C)
\[\text{1}.\text{3}\times \text{1}{{0}^{\text{27}}}\]
done
clear
D)
\[\text{3}.\text{9}\times \text{1}{{0}^{\text{18}}}\]
done
clear
View Answer play_arrow
-
question_answer36) Balmer gives an equation for wavelength of visible radiation of \[{{H}^{-}}\] spectrum as \[\lambda =\frac{k{{n}^{2}}}{{{n}^{2}}-4}\]. The value of k in terms of Rydberg's constant R A is
A)
R
done
clear
B)
4R
done
clear
C)
\[\frac{R}{4}\]
done
clear
D)
\[\frac{4}{R}\]
done
clear
View Answer play_arrow
-
question_answer37) In \[{{\mu }_{e}}\]and \[{{\mu }_{h}}\] are electron and hole mobility. E be the applied electric field, the current density \[\tau \] for intristic semiconductor is equal to
A)
\[{{n}_{i}}e({{\mu }_{e}}+{{\mu }_{h}})E\]
done
clear
B)
\[{{n}_{i}}e({{\mu }_{e}}-{{\mu }_{h}})E\]
done
clear
C)
\[\frac{{{n}_{i}}e({{\mu }_{e}}-{{\mu }_{h}})}{E}\]
done
clear
D)
\[\frac{E}{{{n}_{i}}e({{\mu }_{e}}+{{\mu }_{n}})}\]
done
clear
View Answer play_arrow
-
question_answer38) The KE of the electron in an orbit of radius r in hydrogen atom is (e = electronic charge)
A)
\[\frac{{{e}^{2}}}{r}\]
done
clear
B)
\[\frac{{{e}^{2}}}{2r}\]
done
clear
C)
\[\frac{{{e}^{2}}}{r}\]
done
clear
D)
\[\frac{{{e}^{2}}}{2{{r}^{2}}}\]
done
clear
View Answer play_arrow
-
question_answer39) Three charged particles are collinear and are in equilibrium, then
A)
all the charged particles have the same polarity
done
clear
B)
the equilibrium is unstable
done
clear
C)
all the charged particles cannot have the same polarity
done
clear
D)
Both (b) and (c) are correct
done
clear
View Answer play_arrow
-
question_answer40)
The capacitive time constant of the RC circuit shown in the figure is 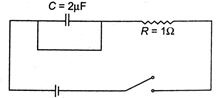
A)
zero
done
clear
B)
infinity
done
clear
C)
2s
done
clear
D)
2us
done
clear
View Answer play_arrow
-
question_answer41) Assertion Mass of moving photon varies inversely as the wavelength. Reason Energy of the particle = Mass x (speed of light\[{{)}^{2}}\]. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer42) Assertion A hollow metallic closed container maintained at a uniform temperature can act as a source of a black body radiation. Reason All metals act as a black body. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer43) Assertion The ratio of inertial mass to gravitational mass is equal to one. Reason The inertial mass and gravitational mass of a body are equivalent. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer44) Assertion In a stationary wave, there is no transfer of energy. Reason There is no outward motion of the disturbance from one particle to adjoining particle in a stationary wave. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer45) Assertion In photoelectron emission the velocity of electron ejected from near the surface is larger than that coming from interior of metal. Reason The velocity of ejected electron will be zero. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer46) Assertion If the ice on the polar caps of the earth melts, then length of day will increase. Reason Moment of inertia of the earth increases, as ice on polar caps melts. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer47) Assertion Dielectric polarisation means formation of positive and negative charges inside the dielectric. Reason Free electron are formed in this process. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer48) Assertion Static crashes are heard on radio, when lightning flash occurs in the sky. Reason Electromagnetic waves having frequency of radiowave range, interfere with radiowaves. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer49) Assertion The satellites equipped with electronic devices are called active satellites. Reason Passive satellite works as active satellite. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer50) Assertion In He-Ne laser, population inversion takes place between energy level of neon atoms. Reason Helium atoms have a meta-stable energy level. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer51) Assertion A transistor amplifier in common emitter configuration has a low input impedance. Reason The base to emitter region is forward biased. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true. Input impedance of common emitter configuration is given by
done
clear
View Answer play_arrow
-
question_answer52) Assertion Thermodynamic process in nature are irreversible. Reason Dissipative effects -cannot be eliminated.- In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer53) Assertion Crystalline solids can cause X-rays to diffract. Reason Interatomic distance in crystalline solids is of the order of 0.1 nm. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer54) Assertion For higher temperature, the peak emission wavelength of a black body shifts to lower wavelength. Reason Peak emission wavelength of a black body is proportional to the fourth power of temperature. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer55) Assertion Displacement of a body may be zero, when distance travelled by it is not zero. Reason The displacement is the longer distance between initial and final position. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer56) Assertion Magnetic field interacts with a moving charge and not with a stationary charge. Reason A moving charge produces a magnetic field. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer57) Assertion There is no current in the metals in the absence of electric field. Reason Motion of free electrons are randomly. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer58) Assertion When height of a tube is less than liquid rise in the capillary tube, the liquid does not over flow. Reason Product of radius of meniscus and height of liquid in capillary tube always remains constant. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer59) Assertion Sound would travel faster on a hot summer day than on cold winter day. Reason Velocity of sound is directly proportional to the square of its absolute temperature. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
question_answer60) Assertion When charges are shared between any two bodies no charge is really lost some loss of energy does occurs. Reason Some energy disappears in the form of heat, sparking etc. In each of the flowing questions, two statements are given. One is portion and the other is reason. Examine the statement carefully and mark the correct answer according to the instruction given below
A)
If both the assertion and reason are true and reason explains the assertion.
done
clear
B)
If both the assertion and reason are true but reason does not explain the assertion.
done
clear
C)
If assertion is true but reason is false.
done
clear
D)
If assertion is false but reason is true.
done
clear
View Answer play_arrow
-
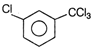
question_answer61)
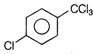
The IUPAC name of the compound 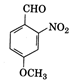
A)
4-methoxy-2-nitro benzaldehyde
done
clear
B)
4-formyl-3-nitro anisole
done
clear
C)
4-methoxy- 6-nitro benzaldehyde
done
clear
D)
2-formyl-5-methoxy nitrobenzene
done
clear
View Answer play_arrow
-
question_answer62) Butyne-1 on oxidation with hot alkaline \[KMn{{O}_{4}}\] would give
A)
\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{COOH}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}COOH+C{{O}_{2}}+{{H}_{2}}O\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}COOH+HCOOH\]
done
clear
View Answer play_arrow
-
question_answer63) Which one of the following statements is false?
A)
Photochemical smog causes irritation in eyes
done
clear
B)
London smog is a mixture of smoke and fog
done
clear
C)
Photochemical smog results in the formation of PAN
done
clear
D)
London smog is oxidising in nature
done
clear
View Answer play_arrow
-
question_answer64) Which of the following aqueous solutions has the highest boiling point?
A)
\[0.1MKN{{O}_{3}}\]
done
clear
B)
\[0.1MN{{a}_{3}}P{{O}_{4}}\]
done
clear
C)
\[0.1M\,BaC{{l}_{2}}\]
done
clear
D)
\[0.1M{{K}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer65) An increase in equivalence conductance of a strong electrolyte with dilution is mainly due to
A)
increase in number of ions
done
clear
B)
increase in ionic mobility of ions
done
clear
C)
increase in both, i.e., number of ions and ionic mobility of ions
done
clear
D)
at normal dilution 100% ionisation of electrolyte
done
clear
View Answer play_arrow
-
question_answer66) The rate constant for a first order reaction becomes six times when the temperature is raised from 350 K to 400 K. Calculate the activation energy for the reaction. \[[R=8.314J{{K}^{-1}}mo{{l}^{-1}}]\]
A)
\[4.17\,KJ\,mo{{l}^{-1}}\]
done
clear
B)
\[41.7\,KJ\,mo{{l}^{-1}}\]
done
clear
C)
\[417.0\,KJ\,mo{{l}^{-1}}\]
done
clear
D)
\[4170\,KJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer67) When dilute aqueous solution of\[AgN{{O}_{3}}\] (excess) is added to \[KI\] solution, positively charged sol of \[Agl\] in formed due to adsorption of
A)
\[NO_{3}^{-}\]
done
clear
B)
\[O_{2}^{-}\]
done
clear
C)
\[A{{g}^{+}}\]
done
clear
D)
\[{{K}^{+}}\]
done
clear
View Answer play_arrow
-
question_answer68) In electrorefining of copper some gold is deposited at
A)
cathode
done
clear
B)
anode mud
done
clear
C)
cathode mud
done
clear
D)
electrode
done
clear
View Answer play_arrow
-
question_answer69) \[CaC{{N}_{2}}+C\] is called on
A)
urea
done
clear
B)
thomas slag
done
clear
C)
nitrolim
done
clear
D)
triple super phosphate
done
clear
View Answer play_arrow
-
question_answer70) Which one of the following forms vortex ring?
A)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[\text{N}{{\text{H}}_{\text{3}}}\]
done
clear
D)
\[{{P}_{4}}{{O}_{10}}\]
done
clear
View Answer play_arrow
-
question_answer71) What is X, in the following reaction? \[KHS{{O}_{4}}+{{F}_{2}}\to HF+X\]
A)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[{{K}_{2}}{{S}_{2}}{{O}_{4}}\]
done
clear
C)
\[{{K}_{2}}{{S}_{2}}{{O}_{3}}\]
done
clear
D)
\[{{K}_{2}}{{S}_{2}}{{O}_{8}}\]
done
clear
View Answer play_arrow
-
question_answer72) Europium is
A)
s- block element
done
clear
B)
p- block element
done
clear
C)
d- block element
done
clear
D)
\[f-\]block element
done
clear
View Answer play_arrow
-
question_answer73) The stability of ferric ion is due to
A)
half-filled d-orbitals
done
clear
B)
half-filled f-orbitals
done
clear
C)
completely filled d-orbitals
done
clear
D)
completely filled f-orbitals
done
clear
View Answer play_arrow
-
question_answer74) An octahedral complex is formed when hybrid orbitals of the following types are involved.
A)
\[\text{s}{{\text{p}}^{\text{3}}}\]
done
clear
B)
\[\text{ds}{{\text{p}}^{\text{2}}}\]
done
clear
C)
\[{{\text{d}}^{\text{2}}}\text{s}{{\text{p}}^{\text{3}}}\]
done
clear
D)
\[\text{s}{{\text{p}}^{\text{3}}}{{d}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer75) Which one amongst of the following isomerism is shown by \[\left[ \text{Pt}{{\left( \text{N}{{\text{H}}_{\text{3}}} \right)}_{\text{2}}}\text{C}{{l}_{\text{2}}} \right]\] ?
A)
Structural
done
clear
B)
Geometrical
done
clear
C)
Optical
done
clear
D)
Conformational
done
clear
View Answer play_arrow
-
question_answer76) What is the structural formula of lithium tetrahydrido aluminate ?
A)
\[Al[Li{{H}_{4}}]\]
done
clear
B)
\[A{{l}_{2}}{{[Li{{H}_{4}}]}_{3}}\]
done
clear
C)
\[Li[Al{{H}_{4}}]\]
done
clear
D)
\[Li{{[Al{{H}_{4}}]}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer77)
Find the major product in the following reaction, 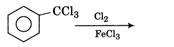
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer78) An organic compound which produces a bluish green coloured flame on heating in presence of copper is
A)
chlorobenzene
done
clear
B)
benzaldehyde
done
clear
C)
aniline
done
clear
D)
benzoic acid
done
clear
View Answer play_arrow
-
question_answer79) In Williamson's synthesis, ethoxy ethane is prepared by
A)
heating sodium ethoxide with ethyl bromide
done
clear
B)
passing ethanol over heated alumina
done
clear
C)
treating ethyl alcohol with excess of cone. \[{{H}_{2}}S{{O}_{4}}\] at 430 - 440 K
done
clear
D)
heating ethanol with dry \[A{{g}_{2}}O\]
done
clear
View Answer play_arrow
-
question_answer80) Which among the following compounds will give a secondry alcohol on reacting with Grignard reagent followed by acid hydrolysis? I. HCHO II. \[{{C}_{2}}{{H}_{5}}CHO\] III.\[\text{C}{{\text{H}}_{\text{3}}}\text{COC}{{\text{H}}_{\text{3}}}\] IV. \[RCOO{{C}_{2}}{{H}_{5}}\] Select the correct answer using the codes given below.
A)
Only II
done
clear
B)
Only III
done
clear
C)
II and IV
done
clear
D)
III and IV
done
clear
View Answer play_arrow
-
question_answer81) Which of the following is the industrial method of preparation of acetaldehyde?
A)
\[C{{H}_{3}}CN\xrightarrow[HCl]{Snc{{l}_{2}}}C{{H}_{3}}CH=NH\] \[\xrightarrow{{{H}_{3}}{{O}^{+}}}C{{H}_{3}}CHO\]
done
clear
B)
\[C{{H}_{3}}COCl+{{H}_{2}}\xrightarrow[BaS{{O}_{4}}]{pd}C{{H}_{3}}CHO+HCl\]
done
clear
C)
\[C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O\xrightarrow{p{{b}^{2+}}}C{{H}_{3}}CHO\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
-
question_answer82) \[{{C}_{3}}{{H}_{6}}O\]did not give a silver mirror with Tollen's reagent, but gave an oxime with hydroxylamine. It can give positive
A)
iodoform test
done
clear
B)
Fehling's test
done
clear
C)
Schiff's test
done
clear
D)
carbylamine test
done
clear
View Answer play_arrow
-
question_answer83) Which of the following carboxylic acids undergoes decarboxylation easily?
A)
\[{{C}_{6}}{{H}_{5}}COCOOH\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}COHC{{H}_{2}}COOH\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}CHOHCOOH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}\underset{\begin{smallmatrix} | \\ N{{H}_{2}} \end{smallmatrix}}{\mathop{CH}}\,COOH\]
done
clear
View Answer play_arrow
-
question_answer84) The stoichiometry of the following reaction is \[{{K}_{2}}{{S}_{2}}{{O}_{8}}(\alpha q)+2KI(\alpha q)\xrightarrow[{}]{{}}\] \[2{{K}_{2}}S{{O}_{4}}(\alpha q)+{{I}_{2}}(\alpha q)\]
A)
\[2:1\]
done
clear
B)
\[\text{1}:2\]
done
clear
C)
\[2:2\]
done
clear
D)
\[1:3\]
done
clear
View Answer play_arrow
-
question_answer85) \[{{\Psi }^{2}}=0\] represents
A)
a node
done
clear
B)
an orbital
done
clear
C)
angular wave function
done
clear
D)
wave function
done
clear
View Answer play_arrow
-
question_answer86) If the de-Brogue wavelength of a particle of mass m is 100 times its velocity then its value in terms of its mass (m) and Planck's constant (h) is
A)
\[\frac{1}{10}\sqrt{\frac{m}{h}}\]
done
clear
B)
\[10\sqrt{\frac{h}{m}}\]
done
clear
C)
\[\frac{1}{10}\sqrt{\frac{h}{m}}\]
done
clear
D)
\[10\sqrt{\frac{m}{h}}\]
done
clear
View Answer play_arrow
-
question_answer87) The pair having similar geometry is
A)
\[PC{{l}_{3}},NH_{4}^{+}0\]
done
clear
B)
\[BeC{{l}_{2}},{{H}_{2}}O\]
done
clear
C)
\[C{{H}_{4}},CC{{l}_{4}}\]
done
clear
D)
\[l{{F}_{5}},P{{F}_{5}}\]
done
clear
View Answer play_arrow
-
question_answer88) The correct order in which the \[OO\] bond length increase is
A)
\[{{H}_{2}}O<{{O}_{2}}<{{O}_{3}}\]
done
clear
B)
\[{{\text{O}}_{3}}<{{H}_{2}}{{\text{O}}_{2}}<{{\text{O}}_{\text{2}}}\]
done
clear
C)
\[{{\text{O}}_{\text{2}}}<{{\text{O}}_{\text{3}}}<{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\]
done
clear
D)
\[{{\text{O}}_{2}}<{{H}_{2}}{{\text{O}}_{2}}<{{\text{O}}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer89) An LPG cylinder, containing 15 kg butane at \[27{}^\circ C\]and 10 atm pressure, is leaking. After one day, its pressure decreased to 8 atm. The quantity of gas leaked is
A)
1 kg
done
clear
B)
2 kg
done
clear
C)
3 kg
done
clear
D)
4 kg
done
clear
View Answer play_arrow
-
question_answer90) Assume each reaction is carried out in a open container. For which reaction\[\Delta H=\Delta E\]?
A)
\[{{H}_{2}}(g)+B{{r}_{2}}(g)\to 2HBr(g)\]
done
clear
B)
\[C(s)+2{{H}_{2}}O(g)\to 2{{H}_{2}}(g)+C{{O}_{2}}(g)\]
done
clear
C)
\[PC{{l}_{5}}(g)\to PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]
done
clear
D)
\[2CO(g)+{{O}_{2}}(g)\to 2C{{O}_{2}}(g)\]
done
clear
View Answer play_arrow
-
question_answer91) In a basic buffer, 0.0025 mole of \[N{{H}_{4}}Cl\] and 0.15 mole of \[N{{H}_{4}}OH\] are present. The pH of the solution will be (\[(p{{K}_{a}})=4.74\].
A)
11.04
done
clear
B)
10.24
done
clear
C)
6.62
done
clear
D)
5.48
done
clear
View Answer play_arrow
-
question_answer92) Strongest conjugate base is
A)
\[\text{C}{{\text{l}}^{-}}\]
done
clear
B)
\[\text{B}{{\text{r}}^{-}}\]
done
clear
C)
\[{{\text{F}}^{-}}\]
done
clear
D)
\[{{\text{I}}^{-}}\]
done
clear
View Answer play_arrow
-
question_answer93) For the gas phase reaction,\[{{C}_{2}}{{H}_{4}}+{{H}_{2}}{{C}_{2}}{{H}_{6}}\,\,;\,\,\,[\Delta H=-32.7Kcal]\]Carried out in a vessel, the equilibrium concentration of \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\]can be increased by
A)
decreasing the pressure
done
clear
B)
increasing tile temperature
done
clear
C)
removing some \[{{\text{C}}_{\text{2}}}{{\text{H}}_{6}}\]
done
clear
D)
adding some \[{{\text{H}}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer94) Which one of the following is a conjugated protein?
A)
Phosphoprotein
done
clear
B)
Glycoprotein
done
clear
C)
Chromoprotein
done
clear
D)
All of these
done
clear
View Answer play_arrow
-
question_answer95) A solution containing 0.319 g of\[\text{CrC}{{\text{l}}_{3}}\text{.6}{{\text{H}}_{2}}O\]was passed through a cation exchange resin and acid coming out of the cation exchange resin required 28.5 mL of 0.125 M\[NaOH\]. Determine correct formula of the complex [mol. wt. of the complex =266.5]
A)
\[[Cr({{H}_{2}}{{O}_{6}})]C{{l}_{3}}\]
done
clear
B)
\[[Cr({{H}_{2}}{{O}_{5}})Cl]{{H}_{2}}O.C{{l}_{2}}\]
done
clear
C)
\[[Cr{{({{H}_{2}}O)}_{4}}C{{l}_{2}}]Cl.2{{H}_{2}}O\]
done
clear
D)
\[[Cr{{({{H}_{2}}O)}_{3}}C{{l}_{3}}]3{{H}_{2}}O\]
done
clear
View Answer play_arrow
-
question_answer96) Which of the following is not an actinoid?
A)
Curium (Z = 96)
done
clear
B)
Californium (Z = 98)
done
clear
C)
Uranium (Z =92)
done
clear
D)
Terbium (Z = 65)
done
clear
View Answer play_arrow
-
question_answer97) The period number in the long form of the periodic table is equal to
A)
magnetic quantum number of any element of the period
done
clear
B)
atomic number of any element of the period
done
clear
C)
maximum principal quantum number of any element of the period
done
clear
D)
maximum azimuthal quanoum numbers of any element of the period
done
clear
View Answer play_arrow
-
question_answer98) If the IP of Na is 5.48 eV, the ionization potential of K will be
A)
same as that of Na
done
clear
B)
4.34 eV
done
clear
C)
5.68 eV
done
clear
D)
10.88 eV
done
clear
View Answer play_arrow
-
question_answer99) The entropy change involved in the isothermal reversible expansion of 2 moles of an ideal gas from a volume of 10 dm3 at \[27{}^\circ C\] is to a volume of \[\text{1}00\text{ d}{{\text{m}}^{\text{3}}}\]
A)
\[42.3J\,mo{{l}^{-1}}{{K}^{-1}}\]
done
clear
B)
\[38.3\,J\,mo{{l}^{-1}}{{K}^{-1}}\]
done
clear
C)
\[35.8\,\,J\,mo{{l}^{-1}}{{K}^{-1}}\]
done
clear
D)
\[32.3\,\,J\,mo{{l}^{-1}}{{K}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer100) Which of the following reagents may by used to distinguish between phenol and benzoic acid?
A)
Neutral \[FeC{{l}_{3}}\]
done
clear
B)
Aqueous \[NaOH\]
done
clear
C)
Tollen's reagent
done
clear
D)
Molisch reagent
done
clear
View Answer play_arrow
-
question_answer101) Assertion Conformers are impractical to separate. Reason Conformers have negligibly small difference in their potential energy.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer102) Assertion p-toluidine is a stronger base than m -toluene. Reason Methyl group from m ?position exerts smaller electron donating inductive (\[+I\]) effect than from p-position.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer103) Assertion 2-butyne on controlled hydrogenation with \[\text{Pd}/\text{CaC}{{\text{O}}_{\text{3}}}\]in presence of \[PbO\] gives cis- 2-butene. Reason Hydrogenation occur at the surfaces of metal containing adsorbed hydrogen.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer104) Assertion Treatment of chloroethane with a saturated solution of \[\text{AgCN}\]gives ethyl isocyanide as the major product. Reason Cyanide (\[\text{C}{{\text{N}}^{\text{-}}}\]) is an ambident nucleophile.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer105) Assertion? Reaction of alcohols with \[SOC{{l}_{2}}\]is catalysed by the presence of a tertiary amine\[({{R}_{2}}N)\] Reason Tertiary amine promote the reaction by reacting with the by-product \[HCl\].
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer106) Assertion Aldol condensation is usually carried out in dilute solution of a strong base. Reason Concentrated solution of strong base involves Canniz zaro reaction.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer107) Assertion Malonic acid \[\left( \text{HOOC }\text{C}{{\text{H}}_{2}}\text{COOH} \right)\] does not form cyclic anhydride on heating. Reason It is like \[\beta \]-keto acid, on heating it prefer to decarboxylate.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer108) Assertion Both 106 g of sodium carbonate and 12 g of graphite have same number of carbon atoms. Reason Both 106 g sodium carbonate and 12 g of graphite contain 1 g-atom of carbon atoms.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer109) Assertion- Energy of electron is largely determined by its principal quantum number. Reason Principal quantum number is a measure of the most possible distance of finding the electron around the nucleus.
A)
Both Assertion and Reason are true and Reason is the correct
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer110) Assertion When 1.0 mol of\[NaCl\]is doped with \[{{10}^{-3}}\] mol \[SrC{{l}_{2}}\], the number of cationic sites remaining vacant is \[{{10}^{-3}}\]. Reason Each\[SrC{{l}_{2}}\] unit produces two cationic vacancy.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer111) Assertion A process for which\[\Delta {{S}_{syst.}}>0\]as \[\Delta H>0\], passes from non-spontaneous to spontaneous state as temperature is increased. Reason At higher temperature, \[T\Delta S\] exceeds\[\Delta H\].
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct expianation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer112) Assertion A catalyst does not influence the value of equilibrium constant. Reason Catalyst influence the rate of both forward and backward reactions equally.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer113) Assertion Addition of a non-volatile solute to a volatile solvent increases the boiling point. Reason Addition of non-volatile solute results in lowering of vapour pressure.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer114) Assertion Electrolysis of molten \[Ca{{H}_{2}}\] produces hydrogen gas at anode. Reason In \[Ca{{H}_{2}}\] hydrogen is present in the form of hydride \[{{H}^{-}}\].
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer115) Assertion\[NaOH\]cannot be stored in a vessel made of aluminium or zinc. Reason A protective layer of oxide is formed on the surface of the metal.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer116) Assertion Boron always forms covalent bond. Reason The small size of \[{{\text{B}}^{\text{3}+}}\]favours formation of covalent bond.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer117) Assertion \[Ca{{F}_{2}}\] has been given the name fluorspar. Reason Solid \[Ca{{F}_{2}}\] emits light when heated.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer118) Assertion The purple colour of \[KMn{{O}_{4}}\] is due to the charge transfer transition. Reason The intense colour in most of the transition metal complexes is due to d-d transition.
A)
Both Assertion and Reason are true and Reason is the correct
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer119) Assertion \[A{{l}_{2}}{{O}_{3}}\] is converted to aluminium by reduction with carbon. Reason Carbon (graphite) has greater affinity for oxygen than Al.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer120) Assertion \[[Ni{{(CO)}_{4}}]\] is a diamagnetic complex. Reason All the electrons in the complex are paired.
A)
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer121) Wings of pigeon, bat and mosquito shows
A)
atavism
done
clear
B)
convergent evolution
done
clear
C)
divergent evolution
done
clear
D)
mutation
done
clear
View Answer play_arrow
-
question_answer122) Gelatin, an important raw material for preparation of photographic emulsion is a by product of
A)
chicken
done
clear
B)
forest
done
clear
C)
cattle
done
clear
D)
fish
done
clear
View Answer play_arrow
-
question_answer123) Phylogenetic system of classification includes
A)
evolutionary trends only
done
clear
B)
genetic trends only
done
clear
C)
evolutionary trend as well as morphology
done
clear
D)
behavioural trends in environment
done
clear
View Answer play_arrow
-
question_answer124) 'Red tide' is caused by
A)
Gonyaulax
done
clear
B)
Ceratium
done
clear
C)
Taceratium
done
clear
D)
All of these
done
clear
View Answer play_arrow
-
question_answer125) A person sitting at rest experiences a temporary cessation of breathing after forced deep breathing for a few minutes. This is due to
A)
too much \[C{{O}_{2}}\] in the blood
done
clear
B)
too much \[{{O}_{2}}\] in the blood
done
clear
C)
very little \[C{{O}_{2}}\] in the blood
done
clear
D)
both high \[{{O}_{2}}\] and very little \[C{{O}_{2}}\] in the blood
done
clear
View Answer play_arrow
-
question_answer126) A physiological response of plants to the duration of light and darkness is a
A)
daily phase cycle
done
clear
B)
circadian rhythms
done
clear
C)
biological clock
done
clear
D)
-photoperiodism
done
clear
View Answer play_arrow
-
question_answer127) ABA is involved in
A)
shoot elongation
done
clear
B)
increased cell division
done
clear
C)
dormancy of seed
done
clear
D)
root elongation'
done
clear
View Answer play_arrow
-
question_answer128) There is increase in blood urea when there is insufficient filtration in
A)
loop of Henie
done
clear
B)
distal tubule
done
clear
C)
Bowman's capsule
done
clear
D)
collecting tubule
done
clear
View Answer play_arrow
-
question_answer129) Aerobic respiration produces more usable chemical energy than fermentation, because fermentation involves
A)
formation of lactic acid
done
clear
B)
complete oxidation of food
done
clear
C)
partial oxidation of food
done
clear
D)
evolution of \[C{{O}_{2}}\] and alcohol
done
clear
View Answer play_arrow
-
question_answer130) Which one of the following correctly describes the location of some body parts in the earthworm (Pheretima) ?
A)
Four pairs of spermathecae in 4-7 segments
done
clear
B)
One pair of ovaries attached at inter-segmental septum of 14th and 15th segments
done
clear
C)
Two pairs of testes in 10th and 11th segments
done
clear
D)
Two pairs of accessory glands in 16-18th Segments
done
clear
View Answer play_arrow
-
question_answer131) Hypothetical plant hormones are
A)
florigen
done
clear
B)
vernalin
done
clear
C)
florigen and vernalin
done
clear
D)
auxin
done
clear
View Answer play_arrow
-
question_answer132) Which one of the following shows heterothallism?
A)
Rhizopus
done
clear
B)
Bacterium
done
clear
C)
Cycas
done
clear
D)
Ricinus
done
clear
View Answer play_arrow
-
question_answer133) A drug addict showed symptoms such as increased appetite, chest pain, redness eyes, increased urination. He was possibily taking
A)
cannabis compounds
done
clear
B)
LSD
done
clear
C)
cocaine
done
clear
D)
amphetamines
done
clear
View Answer play_arrow
-
question_answer134) The brain disease caused due to accumulation of amyloid \[\beta \]-peptide is
A)
Addison's disease
done
clear
B)
Huntington's disease
done
clear
C)
Parkinson's disease
done
clear
D)
Alzheimer's disease
done
clear
View Answer play_arrow
-
question_answer135) Which of the following is a 'cyanophage'?
A)
\[S-13\]
done
clear
B)
\[\phi \times 174\]
done
clear
C)
\[SV-40\]
done
clear
D)
\[LPP-1\]
done
clear
View Answer play_arrow
-
question_answer136)
Match items in Column I with those in | Column II. | Column II |
| A. Peritrichous flagella B. Living fossil C. Rhizophore D. Smallest flowering plant E. Largest pernnial alga | 1. Ginkgo 2. Macrpcystis 3. E.coli 4. Selaginella 5. Wolffia |
A B C D E
A)
3 1 4 5 2
done
clear
B)
2 3 4 1 5
done
clear
C)
4 2 1 5 3
done
clear
D)
2 4 3 5 1
done
clear
View Answer play_arrow
-
question_answer137) Osmosis is a type of
A)
imbibition of solution
done
clear
B)
diffusion of solvent
done
clear
C)
evaporation of water
done
clear
D)
diffusion of solute
done
clear
View Answer play_arrow
-
question_answer138) RQ (Respiratory Quotient) is defined as
A)
volume of \[C{{O}_{2}}\] evolved = volume of \[{{O}_{2}}\]consumed
done
clear
B)
\[\frac{\text{volume of }{{\text{O}}_{2}}\text{ consumed}}{volume\,of\,C{{O}_{2}}volved}\]
done
clear
C)
\[\frac{\text{volume of C}{{\text{O}}_{2}}\text{ evolved}}{volume\,of\,{{O}_{2}}onsumed}\]
done
clear
D)
\[\frac{\text{volume of }{{\text{O}}_{2}}\text{ evolved}}{volume\,of\,C{{O}_{2}}consumed}\]
done
clear
View Answer play_arrow
-
question_answer139) Atretic follicles are found in the
A)
fallopian tubes
done
clear
B)
uterus
done
clear
C)
labia majora
done
clear
D)
ovary
done
clear
View Answer play_arrow
-
question_answer140) Which one is matched correctly?
A)
Arsenic?Black foot disease
done
clear
B)
Flouride?Itai-itai
done
clear
C)
Mercury?Skeletal fluorosis
done
clear
D)
Cadmium?Minamata disease
done
clear
View Answer play_arrow
-
question_answer141) Leghaemoglobin helps in
A)
nitrogen-fixation
done
clear
B)
protecting nitrogenase from \[{{O}_{2}}\]
done
clear
C)
destroy bacteria
done
clear
D)
transport of food in plants
done
clear
View Answer play_arrow
-
question_answer142)
Match the Column I with Column II. | Column I | Column II |
| A. Bulliform cells B. Guard cells C. Lenticels D. Subsidiary cell | 1. Stomata 2. Aerating pore 3. Accessory cells 4. Isobilateral leaf |
A)
A B C D 1 2 3 4
done
clear
B)
3 1 2 4
done
clear
C)
4 1 2 3
done
clear
D)
4 3 2 1
done
clear
View Answer play_arrow
-
question_answer143) 'Cladode' is a characteristic morphological feature of
A)
Asparagus and Ruscus
done
clear
B)
Casuarina and Opuntia
done
clear
C)
Cladophora and Cactus
done
clear
D)
Citrus and Euphorbi'a
done
clear
View Answer play_arrow
-
question_answer144) The species diversity decreases from lower to higher altitudes on a mountain. This is due to
A)
increase in temperature
done
clear
B)
decrease in temperature
done
clear
C)
greater seasonal variability
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
-
question_answer145) What is not a common feature is Periplaneta and Scorpions?
A)
Excretory organs are malpighian tubules
done
clear
B)
No appendages in abdomen
done
clear
C)
Respiratory organs are trachea
done
clear
D)
Both are mostly terrestrial arthropods
done
clear
View Answer play_arrow
-
question_answer146) Cycas is classified as a gymnosperms due to its
A)
motile sperms
done
clear
B)
fruit formation
done
clear
C)
naked ovule
done
clear
D)
phycnoxylic wood
done
clear
View Answer play_arrow
-
question_answer147) In an area, a population with large size individuals having long life span, more parental care and slow development was present. The type of population growth curve will be
A)
S-shaped
done
clear
B)
J-shaped
done
clear
C)
Z-shaped
done
clear
D)
All of these
done
clear
View Answer play_arrow
-
question_answer148) A gland called 'Clock of ageing' that gradually reduces and degenerates in ageing is
A)
thyroid
done
clear
B)
thymus
done
clear
C)
parathyroid
done
clear
D)
pituitary
done
clear
View Answer play_arrow
-
question_answer149) Which of the following statement is correct?
A)
\[DPD=OP-WP\]
done
clear
B)
\[DPD=OP+WP\]
done
clear
C)
\[DPD=WP-OP\]
done
clear
D)
\[DPD=TP+OP\]
done
clear
View Answer play_arrow
-
question_answer150) Censar mechanism of seed dispersal is found in
A)
Papaveraceae
done
clear
B)
Liliaceae
done
clear
C)
Leguminosae
done
clear
D)
Rosaceae
done
clear
View Answer play_arrow
-
question_answer151) At a particular locus, frequency of 'A' allele is 0.6 and that of 'a' is 0.4. What would be the frequency of heterozygotes in a randomly mating population of equilibrium?
A)
0.16
done
clear
B)
0.36
done
clear
C)
0.48
done
clear
D)
0.24
done
clear
View Answer play_arrow
-
question_answer152) The \[{{C}_{4}}\] plants differ from \[{{C}_{3}}\] plants with reference to the
A)
substrate that accepts\[C{{O}_{2}}\]in carbon assimilation
done
clear
B)
type of end product
done
clear
C)
type of pigment involved in photosynthesis
done
clear
D)
number of ATP that are consumed in preparing sugar
done
clear
View Answer play_arrow
-
question_answer153) The colour in the brown fat is due is
A)
its larger capacity for generating heat
done
clear
B)
large number of mitochondria present
done
clear
C)
a high concentration of iron containing cytochrome pigments
done
clear
D)
presence of chromatophores
done
clear
View Answer play_arrow
-
question_answer154) Which of the following is common among mammals?
A)
They do not moult
done
clear
B)
They have seven cervical vertebrae
done
clear
C)
They are carnivores
done
clear
D)
They have ventral nerve cord
done
clear
View Answer play_arrow
-
question_answer155) Elater mechanism for seed dispersal is exhibited by
A)
Riccia
done
clear
B)
Marchantia
done
clear
C)
Dryopteris
done
clear
D)
Funaria
done
clear
View Answer play_arrow
-
question_answer156) In which one of the following pairs, the two items mean the same thing?
A)
Haemophilia?Blood cancer
done
clear
B)
SA-node?Pacemaker
done
clear
C)
Malleus?Anuil
done
clear
D)
Leucocytes?Lymphocytes
done
clear
View Answer play_arrow
-
question_answer157) Which of the following part of human brain is also called emotional brain?
A)
Corpus callosum
done
clear
B)
Limbic system
done
clear
C)
Epithalamus
done
clear
D)
Broca's area
done
clear
View Answer play_arrow
-
question_answer158) Which of the following set of elements are essential for the photosynthesis to occurs
A)
\[\text{Cu},\text{ Co},\text{ Fe}\]
done
clear
B)
\[\text{Cu},\text{ Mo},\text{ Zn}\]
done
clear
C)
\[\text{Mg},\text{ Co},\text{ Mn}\]
done
clear
D)
\[\text{Mg},\text{ Fe},\text{ Mn},\text{ Cu},\text{ Cl},\text{ P}\]
done
clear
View Answer play_arrow
-
question_answer159) The disease caused by Leish mania and transmitted by Phlebotomus is
A)
African sleeping sickness
done
clear
B)
Amoebic dysentery
done
clear
C)
Kala-azar fever
done
clear
D)
Chaga's disease
done
clear
View Answer play_arrow
-
question_answer160) Saheli, is an oral contraceptive pill that has very high contraceptive value with little side effects. It is because
A)
it is taken once in a week
done
clear
B)
it contains synthetic progesterone
done
clear
C)
it contains centchroman
done
clear
D)
it decreases risk of cancer
done
clear
View Answer play_arrow
-
question_answer161) Assertion Rigor mortis is the state of body stiffering prior to death. Reason It is due to relaxation of action and myosin filaments. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer162) Assertion Seeds fails to germinate at very low and high temperature. Reason Seeds sown deep into the soil fail to germinate. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer163) Assertion Lysosomes are organalle in eukaryotic cells that contains digestive enzymes to digest macromolecules. Reason Lysosomes are also called phagolysosomes or heterophagosomes or digestive vacuoles. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer164) Assertion Reproductive isolation brings about sympatric speciation. Reason It is the primary mode of speciation. e.g., Darwin's finches. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer165) Assertion Presence of photorespiration is considered as a wasteful and energy consuming process in crop plants, ultimately leads to reduction in yield. Reason During \[{{C}_{3}}\] synthesis up 50% \[C{{O}_{2}}\]fixed may have to pass through photorespiratory process to form carbohydrate such as sucrose. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer166) Assertion Enzymes becomes inactive below minimum temperature. Reason The inactivity of the enzymes is due to denaturation. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer167) Assertion Fine structure of the objects can be observed by Transmission Electron Microscope (TEM). Reason Study of living cells can not be done through TEM, because of high voltage, which is required to operate it, kills the cells. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer168) Assertion The total content of iron in an adult body is 3.5 gram. The iron deficiency lead to ammonia. Reason Iron (\[F{{e}^{2+}}\]) combines with the pigment porphyrin to form heme. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer169) Assertion Mammals have developed a complex respiratory system. Reason Mammalian skin is impermeable to gases. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer170) Assertion Removel of keystone species cause serious disruption in the functioning of the community. Reason Keystone species are low in abundance (or biomass) than the dominant species. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer171) Assertion In bacteria the chromosome is irregularly folded into a compact mass, the nucleoid or genophore ot definite form. Reason In bacteria there is no organised nucleus. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer172) Assertion DNA is more stable while RNA is more reactive. Reason DNA was first discovered by Watson and Crick (1953). These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer173) Assertion Second infection of the same pathogen is quickly eliminated. Reason Preformed memory B and T-cells elicit a quick and vigorous attack on pathogens. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer174) Assertion Eukaryotic cells have more DNA than prokaryotic cells. Reason Eukaryotes are more complex than prokaryotes genetically. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer175) Assertion In open water zone upto the depth to which light can penetrate, called photic zone. Reason The photic zone is categorised into euphotic and disphotic zone. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer176) Assertion Zoospores in Chlamydomonas are frequently formed in the night during favourable conditions. Reason Zoospore swim for a certain time and then grows into a new plant. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer177) Assertion After hearing a sound, nerve impulse passes from neurons to the brain. Reason The neurons which passes nerve impulse from body organ to brain is called afferent neuron. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer178) Assertion Placenta in addition to connection with mother and foetus to ductless gland. Reason It releases human gonadotropins. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer179) Assertion A woman is capable of sucing a man of refusing to own a child, who has blood group The man has blood group B and woman has A. Reason She is right as genetically, he can be the father of the child. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow
-
question_answer180) Assertion The thallus of Riccia is internally differentiated into an upper photosynthetic region and lower storage region. Reason The lower storage region is formed from parenchymatous cells. These questions consist two statement each printed as Assertion and Reason. While answering these question, you are required to choose any one of the following four options.
A)
Both Assertion, and Reason are true and Reason is the correct explanation of Assertion
done
clear
B)
Both Assertion and Reason are true, but Reason is not the correct explanation of Assertion
done
clear
C)
Assertion is true, but Reason is false
done
clear
D)
Both Assertion and Reason are false
done
clear
View Answer play_arrow