A)
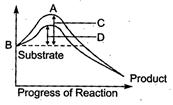
A B C D Potential energy Transition state Activation energy with enzyme Activation energy without enzyme
B)
A B C D Transition energy Potential energy Activation energy without enzyme Activation energy with enzyme
C)
A B C D Potential energy Transition state Activation energy with enzyme Activation energy without enzyme
D)
A B C D Activation energy with enzyme Transition state Activation energy without enzyme Potential energy
Correct Answer: B
Solution :
The amount of energy required to raise the energy of molecules at which chemical reaction can occur is called activation energy. Thus activation energy is actually the energy required to form the transition state. Enzymes dramatically reduce the activation energy of a reaction, so that most molecules can easily get over the activation energy barrier and quickly turn into product. Simply we can say that activation energy of an enzyme catalysed reaction is lower than that of an uncatalysed reaction.You need to login to perform this action.
You will be redirected in
3 sec
