A)
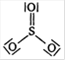
B)
![]()
C)
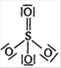
D)
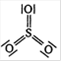
Correct Answer: D
Solution :
Formal charges help in the selection of the lowest energy structure from a number of possible Lewis structures for a given species. Generally the lowest energy structure is the one with the smallest formal charges on the atoms. Formal charge on an atom: = total no. of valence electrons-non-bonding electrons \[-\frac{1}{2}\times \] bonding electrons. For Lewis structure of \[S{{O}_{3}}\]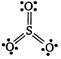 Formal charge on S atom \[=6-0-\frac{1}{2}\times 12=0\] Formal charge on three O atoms \[=6-4-\frac{1}{2}\times 4=0\]
Formal charge on S atom \[=6-0-\frac{1}{2}\times 12=0\] Formal charge on three O atoms \[=6-4-\frac{1}{2}\times 4=0\]
You need to login to perform this action.
You will be redirected in
3 sec
