question_answer 1) In Wiens formula \[{{\lambda }_{m}}\text{T}=\text{b},\] where \[{{\lambda }_{m}}=\]wavelength and T = temperature. The dimensions of Wiens constant b are
A)
\[\left[ {{\text{L}}^{\text{3}}} \right]\]
done
clear
B)
[LT]
done
clear
C)
\[[{{L}^{-1}}]\]
done
clear
D)
\[[L\theta ]\]
done
clear
View Answer play_arrow
question_answer 2) A particle executes simple harmonic motion with an amplitude a. The energy of the particle is half kinetic and half potential, when its displacement is
A)
\[\frac{a}{3}\]
done
clear
B)
\[\frac{a}{2}\]
done
clear
C)
\[\frac{a}{\sqrt{2}}\]
done
clear
D)
\[\frac{a}{2\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 3) A projectile of mass m is thrown with a velocity u making an angle \[60{}^\circ \] with the horizontal, neglecting air resistance, the change in momentum of the departure from A to its arrival at B, to along the vertical directions
A)
\[2\,mu\]
done
clear
B)
\[\sqrt{3}\,mu\]
done
clear
C)
\[\,mu\]
done
clear
D)
\[\frac{\,mu}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 4) A particle experience a constant acceleration for 20 s, after starting from rest, it travels a distance 5i in first 10 s and a distance \[{{s}_{2}}\] in next 10 s, then
A)
\[{{s}_{2}}=\text{ }{{s}_{1}}\]
done
clear
B)
\[{{s}_{2}}=\text{ }2{{s}_{1}}\]
done
clear
C)
\[{{s}_{2}}=\text{ }3{{s}_{1}}\]
done
clear
D)
\[{{s}_{2}}=\text{ }4{{s}_{1}}\]
done
clear
View Answer play_arrow
question_answer 5) A projectile is thrown with initial velocity \[\text{(a}\overset{\hat{\ }}{\mathop{\text{i}}}\,+\text{b}\overset{\hat{\ }}{\mathop{\text{j}}}\,\text{)m}/\text{s}.\]If range of projection is twice the maximum height reached by it, then
A)
\[b=\frac{a}{2}\]
done
clear
B)
\[b=a\]
done
clear
C)
\[b=2a\]
done
clear
D)
\[b=4a\]
done
clear
View Answer play_arrow
question_answer 6) A jet of water with area of cross-section 3 \[\text{c}{{\text{m}}^{\text{2}}}\] strikes a wall at an angle \[\theta =\text{6}0{}^\circ \] to the normal and rebounds elastically from the wall with the same speed. If the speed of water in the jet is 12 m/s, then the force acting on the wall is
A)
\[\text{4}.\text{31}\times \text{1}{{0}^{-1}}\text{ N}\]
done
clear
B)
\[\text{4}.\text{32}\times \text{1}{{0}^{-2}}\text{ N}\]
done
clear
C)
\[\text{4}.\text{32}\times \text{1}{{0}^{-3}}\text{ N}\]
done
clear
D)
43.2 N
done
clear
View Answer play_arrow
question_answer 7) The maximum velocity of a simple harmonic motion represented by \[\left( 100\,t\,+\frac{\pi }{6} \right)m\]is given by
A)
300
done
clear
B)
\[\frac{3\pi }{6}\]
done
clear
C)
100
done
clear
D)
\[\frac{\pi }{6}\]
done
clear
View Answer play_arrow
question_answer 8) A metallic wire of length L metre extends by \[l\]metre when stretched by suspending a weight of Mg to it. Then the mechanical energy stored in the wire is
A)
\[Mgl\]
done
clear
B)
\[\frac{Mgl}{2}\]
done
clear
C)
\[\frac{Mgl}{4}\]
done
clear
D)
\[2Mgl\]
done
clear
View Answer play_arrow
question_answer 9) A boy having a mass of 60 kg, holds in hand a school bag of weight 40 N. With what force the floor will push up on his feet ? \[\text{(g }=\text{ 1}0\text{ m}/{{\text{s}}^{\text{2}}})\]
A)
100 N
done
clear
B)
600 N
done
clear
C)
640 N
done
clear
D)
64 N
done
clear
View Answer play_arrow
question_answer 10) A sphere of mass 2 kg and radius 5 cm is rotating at the rate of 300 rev/min. Then the torque required to stop it in 2si revolutions is
A)
\[\text{1}.\text{6}\times \text{1}{{0}^{\text{5}}}\text{ N}-\text{m}\]
done
clear
B)
\[\text{1}.\text{6}\times \text{1}{{0}^{-\text{4}}}\text{ N}-\text{m}\]
done
clear
C)
\[2.5\times \text{1}{{0}^{-3}}\text{ N}-\text{m}\]
done
clear
D)
\[2.5\times \text{1}{{0}^{-2}}\text{ N}-\text{m}\]
done
clear
View Answer play_arrow
question_answer 11) The gravitational potential energy of a body at a distance r from the centre of the earth is U, then its weight at that point is
A)
\[\frac{U}{{{r}^{2}}}\]
done
clear
B)
\[\frac{U}{r}\]
done
clear
C)
\[Ur\]
done
clear
D)
\[U{{r}^{2}}\]
done
clear
View Answer play_arrow
question_answer 12) A source of sound is moving away from a stationary observer with a speed equal to the speed of sound. The apparent frequency heard by the observer will be
A)
\[{{\text{n}}^{\text{2}}}\]
done
clear
B)
\[\text{2n}\]
done
clear
C)
\[\frac{n}{2}\]
done
clear
D)
unchanged
done
clear
View Answer play_arrow
question_answer 13) One end of a uniform rope of length L and of weight w is attached rigidly to a point in the roof and a weight \[{{w}_{1}}\]is suspended from its lower end. Ifs is the area of cross-section of the \[\frac{3L}{4}\] wire, the stress in the wire at a height from 4 its lower end is
A)
\[\frac{{{w}_{1}}}{s}\]
done
clear
B)
\[\frac{{{w}_{1}}+\frac{w}{4}}{s}\]
done
clear
C)
\[\frac{{{w}_{1}}+\frac{3w}{4}}{s}\]
done
clear
D)
\[\frac{{{w}_{1}}+w}{s}\]
done
clear
View Answer play_arrow
question_answer 14) The excess pressure inside a soap bubble of diameter 1 mm is (surface tension\[=\text{ 6}0\times \text{1}{{0}^{-3}}\text{ N}/\text{m}\])
A)
\[\text{24}0\text{ N}/{{\text{m}}^{\text{2}}}\]
done
clear
B)
\[0.\text{48 N}/{{\text{m}}^{\text{2}}}\]
done
clear
C)
\[\text{48}0\text{ N}/{{\text{m}}^{\text{2}}}\]
done
clear
D)
\[0.\text{24 N}/{{\text{m}}^{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 15) Specific heat of a gas undergoing adiabatic change is
A)
zero
done
clear
B)
infinite
done
clear
C)
positive
done
clear
D)
negative
done
clear
View Answer play_arrow
question_answer 16) In a steady state the temperature of the ends A and B of a 20 cm long rod AB are \[100{}^\circ \] and \[0{}^\circ C\]. The temperature at the point C distant 9 cm from A is
A)
\[45{}^\circ C\]
done
clear
B)
\[55{}^\circ C\]
done
clear
C)
\[60{}^\circ C\]
done
clear
D)
\[65{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 17) The temperature of the ideal gas is increased from \[27{}^\circ C\] to \[972{}^\circ C\]. The root mean square speed of its molecules becomes
A)
half
done
clear
B)
twice
done
clear
C)
four times
done
clear
D)
one- fourth
done
clear
View Answer play_arrow
question_answer 18) A quantity of air (y = 1.4) at \[27{}^\circ C\] is compressed suddenly, the temperature of the air system will
A)
fall
done
clear
B)
rise
done
clear
C)
remain unchanged
done
clear
D)
first rise and then fall
done
clear
View Answer play_arrow
question_answer 19) The ends of two rods of different material with their thermal conductivities, radii of cross-sections and lengths all in the ratio 1: 2 are maintained at the same temperature difference, if the rate of flow of heat in the larger rod is 4 cal/s, then rate of flow of heat in the shorter rod will be
A)
1 cal/s
done
clear
B)
2 cal/s
done
clear
C)
8 cal/s
done
clear
D)
16 cal/s
done
clear
View Answer play_arrow
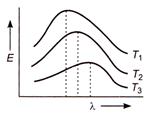
question_answer 20)
Energy emitted by a black body and wavelength relations at temperature T^K, \[{{T}_{3}}K,\]and \[{{\text{T}}_{\text{3}}}\text{K},\]are shown by curves. The relation between the temperatures is
A)
\[{{\text{T}}_{\text{3}}}>{{T}_{2}}>{{T}_{1}}\]
done
clear
B)
\[{{\text{T}}_{1}}>{{T}_{2}}>{{T}_{3}}\]
done
clear
C)
\[{{\text{T}}_{1}}={{T}_{2}}={{T}_{3}}\]
done
clear
D)
\[{{\text{T}}_{1}},{{T}_{2}},{{T}_{3}}\]bear no relation
done
clear
View Answer play_arrow
question_answer 21) The temperature of a furnace is \[2327{}^\circ C\] and the intensity of maximum in its spectrum is nearly at \[12000\overset{\text{o}}{\mathop{\text{A}}}\,\]. If the intensity in the spectrum of a star is maximum nearly at \[4800\overset{\text{o}}{\mathop{\text{A}}}\,\], then the surface temperature of the star is
A)
\[767{}^\circ C\]
done
clear
B)
\[1040{}^\circ C\]
done
clear
C)
\[6500{}^\circ C\]
done
clear
D)
\[6227{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 22) A wheel of radius 1 m rolls forward half a revolution on a horizontal ground. The magnitude of the displacement of the point of wheel initially in contact with the ground is
A)
\[2\pi m\]
done
clear
B)
\[\sqrt{2}\pi m\]
done
clear
C)
\[\sqrt{{{\pi }^{2}}+4}m\]
done
clear
D)
\[\pi \,m\]
done
clear
View Answer play_arrow
question_answer 23) A wave of frequency 500 Hz, travels between X and y, a distance of 600 m in 2 s. How many wavelength are there in distance XY ?
A)
1000
done
clear
B)
300
done
clear
C)
180
done
clear
D)
2000
done
clear
View Answer play_arrow
question_answer 24) The value of current at resonance in series LCR circuit is affected by the value of
A)
R only
done
clear
B)
C only
done
clear
C)
L only
done
clear
D)
R, C and L
done
clear
View Answer play_arrow
question_answer 25) In Youngs double slit experiment, the separation between the slits is made 3 fold, the fringe width becomes
A)
\[\frac{1}{3}Times\]
done
clear
B)
\[\frac{1}{9}Times\]
done
clear
C)
\[3\,Times\]
done
clear
D)
\[9\,Times\]
done
clear
View Answer play_arrow
question_answer 26) A convex lens of focal length 20 cm produces a real image twice the size of the object. Then the distance of the object from the lens is
A)
10 cm
done
clear
B)
20 cm
done
clear
C)
30 cm
done
clear
D)
60 cm
done
clear
View Answer play_arrow
question_answer 27) The speed of light in vacuum is \[\text{3}\times \text{l}{{0}^{\text{8}}}\text{ m}/\text{s}\]and the speed of light in a medium is \[\text{1}.\text{25}\times \text{l}{{0}^{\text{8}}}\text{ m}/\text{s}.\] Then the refractive index of medium is
A)
1.5
done
clear
B)
0.42
done
clear
C)
2.4
done
clear
D)
3.75
done
clear
View Answer play_arrow
question_answer 28) What is the focal length of a double convex lens for which the radius of curvature of each surface is 60 cm ? (n = 1.5)
A)
30 cm
done
clear
B)
60 cm
done
clear
C)
90 cm
done
clear
D)
30 cm
done
clear
View Answer play_arrow
question_answer 29) The ratio of maximum and minimum intensities in an interference pattern is 36 1. The ratio of the amplitudes of the two interfering waves will be
A)
5 : 7
done
clear
B)
7 : 4
done
clear
C)
4 : 7
done
clear
D)
7 : 5
done
clear
View Answer play_arrow
question_answer 30) A slide projector gives a magnification 10. If a slide of dimensions 3 cm x 2 cm is projected on the screen, then the area of image screen is
A)
\[\text{6}000\text{c}{{\text{m}}^{\text{2}}}\]
done
clear
B)
\[\text{6}00\text{c}{{\text{m}}^{\text{2}}}\]
done
clear
C)
\[\text{6}0\text{ c}{{\text{m}}^{\text{2}}}\]
done
clear
D)
\[\text{6 c}{{\text{m}}^{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 31) The focal length of the objective of a telescope is 60 cm. To obtain a magnification of 20 for the relaxed eye, the length of telescope should be
A)
80 cm
done
clear
B)
40 cm
done
clear
C)
63 cm
done
clear
D)
57 cm
done
clear
View Answer play_arrow
question_answer 32) An electron is accelerated at 10 kV to a tungsten target. The wavelength of X-ray photon produced will be
A)
\[0.62\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[2.48\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.124\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1.24\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 33) Photoelectric threshold wavelength for tungsten is 2300 A. Work function for it will be
A)
5.38 eV
done
clear
B)
53.9 eV
done
clear
C)
\[5.39\times {{10}^{-8}}eV\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 34) One nuclear fission release 200 MeV energy. The number of fissions per second which will produce 10 MW of power by a generator of efficiency 25% will be
A)
\[\text{1}.\text{25}\times \text{l}{{0}^{\text{19}}}\]
done
clear
B)
\[\text{1}.\text{25}\times \text{l}{{0}^{\text{18}}}\]
done
clear
C)
\[\text{3}.\text{125}\times \text{1}{{0}^{11}}\]
done
clear
D)
\[\text{5}\times \text{l}{{0}^{\text{11}}}\]
done
clear
View Answer play_arrow
question_answer 35) The ratio of the radius of the orbit for the electron orbiting the hydrogen nucleus to that of an electron orbiting a deuterium nucleus is
A)
1 : 1
done
clear
B)
1 : 2
done
clear
C)
2 : 1
done
clear
D)
1 : 3
done
clear
View Answer play_arrow
question_answer 36) The relation between the half-life T and mean life t of a radioactive sample is
A)
T = 0.693 t
done
clear
B)
t = 0.693 T
done
clear
C)
t = t
done
clear
D)
t = 2.718 T
done
clear
View Answer play_arrow
question_answer 37) Which of the following techniques is concerned with ultrasonic waves?
A)
X-ray photography
done
clear
B)
Sonography
done
clear
C)
CATSCAN
done
clear
D)
ECG
done
clear
View Answer play_arrow
question_answer 38) The average energy released per fission is
A)
108 MeV
done
clear
B)
200 MeV
done
clear
C)
1000 MeV
done
clear
D)
more than 100 MeV
done
clear
View Answer play_arrow
question_answer 39) In a common base circuit, the collector current changes by 0.04 mA when the collector-base voltage is changed by 0.5 V. Output resistance (in \[k\Omega \]) is
A)
6
done
clear
B)
20
done
clear
C)
16
done
clear
D)
12.5
done
clear
View Answer play_arrow
question_answer 40) Three capacitors of capacitances 3\[\mu \]F, 9\[\mu \]F and 18\[\mu \]F are connected once in series and another time in parallel. The ratio of equivalent capacitances in the two cases will be
A)
1 : 15
done
clear
B)
15 : 1
done
clear
C)
1 : 1
done
clear
D)
1 : 3
done
clear
View Answer play_arrow
question_answer 41)
The equivalent capacitance between points A and B of circuit shown in the figure is
A)
\[9\mu F\]
done
clear
B)
\[1\mu F\]
done
clear
C)
\[4.5\mu F\]
done
clear
D)
\[6\mu F\]
done
clear
View Answer play_arrow
question_answer 42) 1000 drops of water each of radius r and charged to a potential V coalesce together to form a big drop. The potential of big drop will be
A)
10 V
done
clear
B)
100 V
done
clear
C)
1000 V
done
clear
D)
\[\frac{V}{100}\]
done
clear
View Answer play_arrow
question_answer 43) Two cells, each of emf£ and internal resistance r are connected in parallel across a resistance R. The power delivered to R is maximum when
A)
\[r=R\]
done
clear
B)
\[R=\frac{r}{2}\]
done
clear
C)
\[R=2\,r\]
done
clear
D)
\[R=0\]
done
clear
View Answer play_arrow
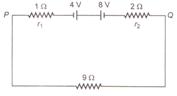
question_answer 44)
Two batteries of emf 4 V and 8 V with internal resistances 1\[\Omega \] and 2\[\Omega \] are connected in a circuit with a resistance of 9\[\Omega \] as shown in figure. The current and potential difference between the points P and Q are
A)
\[\frac{1}{3}A\,\,and\,\,3V\]
done
clear
B)
\[\frac{1}{6}A\,\,and\,\,4V\]
done
clear
C)
\[\frac{1}{9}A\,\,and\,\,9V\]
done
clear
D)
\[\frac{1}{2}A\,\,and\,\,12V\]
done
clear
View Answer play_arrow
question_answer 45) In a potentiometer experiment the galvanometer shows no deflection when a cell is connected across 60 cm of the potentiometer wire. If the cell is shunted by a resistance of 6 0 the balance is obtained across 50 cm of the wire. The internal resistance of the cell is
A)
0.5\[\Omega \]
done
clear
B)
0.6\[\Omega \]
done
clear
C)
1.2\[\Omega \]
done
clear
D)
1.5\[\Omega \]
done
clear
View Answer play_arrow
question_answer 46) The minimum resistance that can be obtained by connecting 5 resistances of \[\frac{1}{4}\Omega \] each, is 4
A)
\[\frac{4}{5}\Omega \]
done
clear
B)
\[\frac{5}{4}\Omega \]
done
clear
C)
\[20\,\Omega \]
done
clear
D)
\[0.05\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 47) A proton, electron and an a-particle is accelerated through the same potential difference enter a region of uniform magnetic field, moving at right angles to the magnetic field B. The ratio of their kinetic energies is
A)
2 1 1
done
clear
B)
2 2 1
done
clear
C)
1 2 1
done
clear
D)
1 1 2
done
clear
View Answer play_arrow
question_answer 48) A current loop of area 0.01 m2 and carrying acurrent of 10 A is held perpendicular to a magnetic field of intensity 0.1 T. The torque acting on the loop (in N-m) is
A)
1.1
done
clear
B)
0.8
done
clear
C)
0.001
done
clear
D)
0.01
done
clear
View Answer play_arrow
question_answer 49) The alternating voltage and current in an electric circuit are respectively given by \[E=100\,\,\sin \,100\,\pi t\] \[\text{I }=\text{ 5 sin 100}\pi \text{t}\] The reactance of the circuit will be
A)
200
done
clear
B)
0.050
done
clear
C)
10
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 50) A uniform metallic wire has a constant voltage across it. The heat produced is double, if
A)
both length and radius of the wire are halved
done
clear
B)
both length and radius of the wire are doubled
done
clear
C)
only radius is doubled
done
clear
D)
only length is doubled
done
clear
View Answer play_arrow
question_answer 51) The quantum number which is responsible for the size of electron cloud is
A)
spin
done
clear
B)
azimuthal
done
clear
C)
principal
done
clear
D)
magnetic
done
clear
View Answer play_arrow
question_answer 52) To deposit one equivalent weight of silver at cathode the charge required will be
A)
\[9.65\times {{10}^{4}}C\]
done
clear
B)
\[9.65\times {{10}^{3}}C\]
done
clear
C)
\[9.65\times {{10}^{5}}C\]
done
clear
D)
\[9.65\times {{10}^{7}}C\]
done
clear
View Answer play_arrow
question_answer 53) ?Electrons are filled in energy orbitals, in increasing order of energy.? This statement is related to
A)
Plancks rule
done
clear
B)
Hunds rule
done
clear
C)
Faults rule
done
clear
D)
Aufbau principle
done
clear
View Answer play_arrow
question_answer 54) Ascorbic acid is a
A)
enzyme
done
clear
B)
vitamin
done
clear
C)
protein
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 55) The outer electronic configuration of transitional elements is
A)
\[(n-1)\,{{s}^{2}}n{{d}^{1-5}}\]
done
clear
B)
\[(n+1)\,{{s}^{2}}n{{d}^{1-5}}\]
done
clear
C)
\[(n+1)\,{{s}^{2}}{{p}^{6}}(n-1)\,{{d}^{1-10}},n{{s}^{0-2}}\]
done
clear
D)
\[n{{s}^{2}}(n+1)\,{{d}^{1-10}}\]
done
clear
View Answer play_arrow
question_answer 56) Strongest base is
A)
RbOH
done
clear
B)
LiOH
done
clear
C)
KOH
done
clear
D)
NaOH
done
clear
View Answer play_arrow
question_answer 57) If the pH of an aqueous solution is 4.7, then its \[{{H}^{+}}\] concentration will be
A)
\[3\times {{10}^{-6}}\]
done
clear
B)
\[3\times {{10}^{-5}}\]
done
clear
C)
\[2\times {{10}^{-5}}\]
done
clear
D)
\[5\times {{10}^{-5}}\]
done
clear
View Answer play_arrow
question_answer 58) Conjugated double bond is present in
A)
butylene
done
clear
B)
butadiene
done
clear
C)
isobutylene
done
clear
D)
propylene
done
clear
View Answer play_arrow
question_answer 59) A radioactive element has a half-life of 10 yr. What percentage of the original amount of it would you expect to remain after 20 yr?
A)
12.5
done
clear
B)
8
done
clear
C)
0
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 60) Given, \[{{H}_{2}}O{{H}_{3}}{{O}^{+}}+{{F}^{-}}\] \[{{F}^{-}}+{{H}_{2}}OHF+O{{H}^{-}}\] Which of the following relation is correct?
A)
\[{{K}_{b}}={{K}_{w}}\]
done
clear
B)
\[{{K}_{b}}\times {{K}_{b}}={{K}_{w}}\]
done
clear
C)
\[\frac{{{K}_{a}}}{{{K}_{b}}}={{K}_{w}}\]
done
clear
D)
\[{{K}_{b}}=\frac{1}{{{K}_{w}}}\]
done
clear
View Answer play_arrow
question_answer 61) The allowed values of m for \[l=2\] is
A)
\[0,\pm 1\]
done
clear
B)
\[\pm \,\,1,\,\,\pm 2\]
done
clear
C)
\[0,\pm \,\,1,\,\,\pm 2\]
done
clear
D)
\[0,\,\,\pm 2\]
done
clear
View Answer play_arrow
question_answer 62) Alkaline dilute \[KMn{{O}_{4}}\] solution is called
A)
Etard reagent
done
clear
B)
Benedict reagent
done
clear
C)
Mullikans reagent
done
clear
D)
Baeyefs reagent
done
clear
View Answer play_arrow
question_answer 63) 2 moles of \[PC{{l}_{5}}\] is heated in one litre vessel. At equilibrium 0.4 mole \[C{{l}_{2}}\] is formed. The value of equilibrium constant will be
A)
\[1\times {{10}^{-3}}\]
done
clear
B)
\[1\times {{10}^{-2}}\]
done
clear
C)
\[2\times {{10}^{-1}}\]
done
clear
D)
\[1\times {{10}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 64) If 19 is the atomic number of an element, then this element will be
A)
a metal with +3 oxidation state
done
clear
B)
a metal with +1 oxidation state
done
clear
C)
an inert gas
done
clear
D)
a metal with -3 oxidation state
done
clear
View Answer play_arrow
question_answer 65) At \[{{25}^{o}}C\] the solubility of sparingly soluble binary salt \[AgCl\] is \[1.25\times {{10}^{-5}}\]mol/L. What will be the value of \[{{K}_{sp}}\] at the same temperature?
A)
\[1.25\times {{10}^{-5}}\]
done
clear
B)
\[1.56\times {{10}^{-10}}\]
done
clear
C)
\[1.\times {{10}^{-10}}\]
done
clear
D)
\[1.44\times {{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 66) Nesslefs reagent is used to detect
A)
\[P{{O}_{4}}^{3-}\]
done
clear
B)
\[MN{{O}_{4}}^{-}\]
done
clear
C)
\[N{{H}_{4}}^{+}\]
done
clear
D)
\[Cr{{O}_{4}}^{2-}\]
done
clear
View Answer play_arrow
question_answer 67) The correct increasing order of electron affinity is
A)
Be, B, N, C
done
clear
B)
C, N, B, Be
done
clear
C)
Be, N, B, C
done
clear
D)
Be, B, C, N
done
clear
View Answer play_arrow
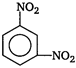
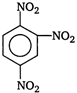
question_answer 68) Nitrobenzene, on reaction with fuming nitric acid at \[{{90}^{o}}C\], the product is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 69) The oxidation number of C in \[C{{H}_{4}},C{{H}_{3}}Cl,C{{H}_{2}}C{{l}_{2}},CHC{{l}_{3}}\] and \[CC{{l}_{4}}\] is respectively
A)
-4, -2, 0, +2, +4
done
clear
B)
+2, 4, 0, -2, -4
done
clear
C)
4, 2, 0, -2, -4
done
clear
D)
0, 2, -2, 4, 4
done
clear
View Answer play_arrow
question_answer 70) Latent heat of vaporisation of a liquid at 500 K and 1 atm pressure is 10.0 kcal/mol. What will be the change in internal energy of 3 moles of liquid at same temperature?
A)
-13.0 kcal
done
clear
B)
-27.0 kcal
done
clear
C)
27.0 kcal
done
clear
D)
13.0 kcal
done
clear
View Answer play_arrow
question_answer 71) If LPG cylinder contains mixture of butane and isobutane, then the amount of oxygen that would be required for combustion of 1 kg of it will be
A)
2.7 kg
done
clear
B)
1.8 kg
done
clear
C)
3.58 kg
done
clear
D)
4.5 kg
done
clear
View Answer play_arrow
question_answer 72) The oxidation number of Cr in \[Cr{{O}_{2}}C{{l}_{2}}\] is
A)
+ 2
done
clear
B)
+ 3
done
clear
C)
+ 4
done
clear
D)
+ 6
done
clear
View Answer play_arrow
question_answer 73) lonisation energy is highest in
A)
\[[Ne]\,\,3{{s}^{1}}\]
done
clear
B)
\[[Ne]\,\,3{{s}^{2}}3{{p}^{3}}\]
done
clear
C)
\[[Ne]\,\,3{{s}^{10}},4{{s}^{2}},4{{p}^{3}}\]
done
clear
D)
\[[Ne]\,\,3{{s}^{2}}3{{p}^{4}}\]
done
clear
View Answer play_arrow
question_answer 74) In the following molecules, the hybrid state at 1 and 3 carbon atoms is \[\overset{1}{\mathop{C}}\,{{H}_{2}}=\overset{2}{\mathop{C}}\,=\overset{3}{\mathop{C}}\,H-\overset{4}{\mathop{C}}\,{{H}_{3}}\]
A)
\[s{{p}^{2}}\]
done
clear
B)
\[s{{p}^{3}}\]
done
clear
C)
\[s{{p}^{3}}d\]
done
clear
D)
sp
done
clear
View Answer play_arrow
question_answer 75) The IUPAC name of \[{{(C{{H}_{3}})}_{3}}C-CH=C{{H}_{2}}\] is
A)
vinyl trimethyl methane
done
clear
B)
2, 2-dimethyl-3-butene
done
clear
C)
tertiary butyl ethane
done
clear
D)
3, 3-dimethyl-l-butene
done
clear
View Answer play_arrow
question_answer 76) The number of \[\sigma \] and n bonds in\[C{{H}_{2}}=CH-CH=C{{H}_{2}}\]
A)
\[8\sigma \] and \[2\pi \] bonds
done
clear
B)
\[9\sigma \] and \[1\pi \] bond
done
clear
C)
\[9\sigma \]and \[3\pi \] bonds
done
clear
D)
\[9\sigma \] and \[2\pi \] bonds
done
clear
View Answer play_arrow
question_answer 77) \[CuS{{O}_{4}}\] dissolves in \[N{{H}_{3}}\]due to formation of
A)
\[Cu{{(OH)}_{2}}\]
done
clear
B)
\[[Cu{{(N{{H}_{3}})}_{4}}{{(OH)}_{2}}]\]
done
clear
C)
\[[Cu{{(N{{H}_{3}})}_{4}}]S{{O}_{4}}\]
done
clear
D)
\[CuO\]
done
clear
View Answer play_arrow
question_answer 78) Phenacetin is used as
A)
antiseptic
done
clear
B)
analgesic
done
clear
C)
antipyretic
done
clear
D)
antimalarial
done
clear
View Answer play_arrow
question_answer 79) The highest magnetic moment will be shown by
A)
Ni
done
clear
B)
Co
done
clear
C)
Fe
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 80) Gypsum is
A)
\[CaS{{O}_{4}}.\,2{{H}_{2}}O\]
done
clear
B)
\[CaC{{O}_{3}}.\,2{{H}_{2}}O\]
done
clear
C)
\[CaS{{O}_{4}}.\,1/2{{H}_{2}}O\]
done
clear
D)
\[Si{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 81) Propene \[\xrightarrow{HBr}\,\,A\xrightarrow[\left( ii \right)\text{ }{{H}_{2}}O/boil]{\left( i \right)Mg/ether}B\]In the above sequence of reactions B is
A)
propane
done
clear
B)
butane
done
clear
C)
propene
done
clear
D)
ethane
done
clear
View Answer play_arrow
question_answer 82) Which is a characteristic of a catalyst?
A)
It changes the equilibrium point
done
clear
B)
It initiates the reaction
done
clear
C)
It alters the rate of a reaction
done
clear
D)
It increases the average kinetic energy of the molecules
done
clear
View Answer play_arrow
question_answer 83) 83. The product \[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-CHC{{l}_{2}}\] is formed when \[HOCl\] reacts with
A)
\[C{{H}_{3}}-C{{H}_{2}}-C\equiv CH\]
done
clear
B)
\[C{{H}_{3}}-C\equiv CH\]
done
clear
C)
\[C{{H}_{3}}-C\equiv C-C{{H}_{3}}\]
done
clear
D)
\[CH\equiv CH\]
done
clear
View Answer play_arrow
question_answer 84) Which of the following is an electrophile?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[AlC{{l}_{3}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 85) A sample of radioactive substance with a \[{{t}_{1/2}}=3\] days was found to contain 3g of it, when received exactly after 12 days. The amount of radioactive substance when it was sealed
A)
48 g
done
clear
B)
12 g
done
clear
C)
24 g
done
clear
D)
6g
done
clear
View Answer play_arrow
question_answer 86) The correct electronic configuration of Cu (29)
A)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}},4{{s}^{1}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{5}}3{{d}^{11}},4{{s}^{1}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{9}},4{{s}^{1}}\]
done
clear
D)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{5}}3{{d}^{10}},4{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 87) Which enzyme is effective in the following reaction? \[{{C}_{6}}{{H}_{12}}{{O}_{6}}\xrightarrow{{}}2{{C}_{2}}{{H}_{5}}OH+2C{{O}_{2}}\]
A)
Invertase
done
clear
B)
Zymase
done
clear
C)
Maltase
done
clear
D)
a-amylase
done
clear
View Answer play_arrow
question_answer 88) Which of the following is a buffer?
A)
\[N{{H}_{4}}OH+N{{H}_{4}}Cl\]
done
clear
B)
\[NaOH+N{{a}_{2}}S{{O}_{4}}\]
done
clear
C)
\[NaOH+C{{H}_{3}}COONa\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}+{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 89) The most electronegative element in periodic table is
A)
Fr
done
clear
B)
F
done
clear
C)
He
done
clear
D)
N
done
clear
View Answer play_arrow
question_answer 90) Cryolite is the ore of
A)
Fe
done
clear
B)
Cu
done
clear
C)
Ag
done
clear
D)
Al
done
clear
View Answer play_arrow
question_answer 91) Which of the following is not a member of 3d-transition series?
A)
Fe
done
clear
B)
Co
done
clear
C)
Au
done
clear
D)
Cu
done
clear
View Answer play_arrow
question_answer 92) The solubility of \[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}\] is s mol/L. Its solubility product is
A)
\[72\,{{s}^{5}}\]
done
clear
B)
\[108\,{{s}^{5}}\]
done
clear
C)
\[9\,{{s}^{2}}\]
done
clear
D)
\[8\,{{s}^{3}}\]
done
clear
View Answer play_arrow
question_answer 93) A group 16 element exists in the monoatomic state in the metallic lattice. It also exists in two crystalline forms. The metal is
A)
S
done
clear
B)
Po
done
clear
C)
Se
done
clear
D)
Te
done
clear
View Answer play_arrow
question_answer 94) The bond energies of \[{{F}_{2}},C{{l}_{2}},B{{r}_{2}}\] and \[{{I}_{2}}\] are 155, 244, 193 and 151 kJ/mol. The weakest bond will be
A)
\[C{{l}_{2}}\]
done
clear
B)
\[B{{r}_{2}}\]
done
clear
C)
\[{{I}_{2}}\]
done
clear
D)
\[{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 95) The process of converting hydrated alumina into anhydrous alumina is called
A)
smelting
done
clear
B)
roasting
done
clear
C)
calcination
done
clear
D)
dressing
done
clear
View Answer play_arrow
question_answer 96) If the hydrogen ion concentration in an acid is \[{{10}^{-8}}\] mol/L then the pH
A)
9
done
clear
B)
7
done
clear
C)
< 7
done
clear
D)
> 8
done
clear
View Answer play_arrow
question_answer 97) Tischenko reaction proceeds in the presence of
A)
Zn and \[ZnC{{l}_{2}}\]
done
clear
B)
Al powder
done
clear
C)
\[Al{{(O{{C}_{2}}{{H}_{5}})}_{3}}\] and anhydrous \[AlC{{l}_{3}}\]
done
clear
D)
\[FeC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 98) Which is most soluble in water?
A)
\[AgBr\]
done
clear
B)
\[AgCl\]
done
clear
C)
\[AgF\]
done
clear
D)
\[AgI\]
done
clear
View Answer play_arrow
question_answer 99) Blue vitriol is
A)
\[MgS{{O}_{4}}.\,7{{H}_{2}}O\]
done
clear
B)
\[CuS{{O}_{4}}.5{{H}_{2}}O\]
done
clear
C)
\[FeS{{O}_{4}}.7{{H}_{2}}O\]
done
clear
D)
\[CaS{{O}_{4}}.\,2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 100) The silver is extracted by Parkes process. The basis of this method is
A)
silver is immiscible in molten Zn
done
clear
B)
Ag is miscible in NaCN
done
clear
C)
Ag is more miscible in molten Zn than in molten Pb
done
clear
D)
Ag is more miscible in molten Pb in comparison to molten Zn
done
clear
View Answer play_arrow
question_answer 101) Fright and flight hormone is secreted by
A)
pituitary gland
done
clear
B)
pineal gland
done
clear
C)
adrenal gland
done
clear
D)
thyroid gland
done
clear
View Answer play_arrow
question_answer 102) Glissons capsules are found in
A)
liver
done
clear
B)
kidney
done
clear
C)
pancreas
done
clear
D)
intestinal wall
done
clear
View Answer play_arrow
question_answer 103) Certain RNA viruses carry a gene for an enzyme that uses viral RNA as a template in the synthesis of DNA. This enzyme is
A)
viral nuclease
done
clear
B)
RNA replicase
done
clear
C)
RNA polymerase
done
clear
D)
reverse transcriptase
done
clear
View Answer play_arrow
question_answer 104) Which of the following disorder is associate with chronic alcoholism and malnutrition?
A)
Hobnail liver
done
clear
B)
Amyloid liver
done
clear
C)
Laennecs cirrhosis
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 105) \[S{{O}_{2}}\]pollution is indicated by
A)
grasses
done
clear
B)
mosses
done
clear
C)
lichens
done
clear
D)
fossils
done
clear
View Answer play_arrow
question_answer 106) Hygroscopic roots are found in
A)
Bryophyllum
done
clear
B)
Vanda
done
clear
C)
Khizophora
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 107) Which of the following blood groups is universal donor?
A)
A
done
clear
B)
B
done
clear
C)
AB
done
clear
D)
O
done
clear
View Answer play_arrow
question_answer 108) Polytene chromosomes are found in
A)
muscles
done
clear
B)
salivary glands
done
clear
C)
connective tissue
done
clear
D)
endocrine glands
done
clear
View Answer play_arrow
question_answer 109) Biometric genetics deals with
A)
die biochemical explanations of various genetical phenomena
done
clear
B)
the effect of environment on genetic makeup of organisms
done
clear
C)
the genetical radiations on the living organisms
done
clear
D)
the inheritance of quantitative traits
done
clear
View Answer play_arrow
question_answer 110) The spread of AIDS is promoted by
A)
homosexuality
done
clear
B)
immoral way of life
done
clear
C)
use of infected needles in blood transfusion
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 111) The study of life forms and environment of past ages is called
A)
Ecology
done
clear
B)
Geoecology
done
clear
C)
Palaeoecology
done
clear
D)
Neoecology
done
clear
View Answer play_arrow
question_answer 112) Entamoeba differs from Amoeba in
A)
having a large number of contractile vacuoles
done
clear
B)
having two contractile vacuoles
done
clear
C)
having no contractile vacuole
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 113) Inulin is a polymer of
A)
glucose
done
clear
B)
fructose
done
clear
C)
lactose
done
clear
D)
galactose
done
clear
View Answer play_arrow
question_answer 114) Which of the following is most abundan enzymatic protein in plants?
A)
Ribozyme
done
clear
B)
Rubisco
done
clear
C)
Pepsin
done
clear
D)
Yeast extract
done
clear
View Answer play_arrow
question_answer 115) The interval between infection and appearance of disease is known as
A)
incubation period
done
clear
B)
inoculation
done
clear
C)
penetration
done
clear
D)
infection period
done
clear
View Answer play_arrow
question_answer 116) \[\alpha \]- helical model of protein was proposed by
A)
Pauling and Corey
done
clear
B)
Stanley
done
clear
C)
Webster
done
clear
D)
Robert Brown
done
clear
View Answer play_arrow
question_answer 117) Hurlers disease is caused by
A)
absence of lysosome
done
clear
B)
absence of reductase
done
clear
C)
absence of lomasomes
done
clear
D)
absence of \[\alpha \]-protein
done
clear
View Answer play_arrow
question_answer 118) Which of the following is anucleated living plant cell?
A)
Sieve tube
done
clear
B)
Vessel
done
clear
C)
Tracheid
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 119) Oil storing plastids are called
A)
amyloplast
done
clear
B)
elaioplast
done
clear
C)
chromoplast
done
clear
D)
chloroplast
done
clear
View Answer play_arrow
question_answer 120) Which of the following cells of Leucosotenia sponge is self replicating and capable of giving rise to all other types of cells?
A)
Amoebocyte
done
clear
B)
Chromocyte
done
clear
C)
Choanocyte
done
clear
D)
Collenocyte
done
clear
View Answer play_arrow
question_answer 121) Incomplete dominance is shown by
A)
Pisum sativum
done
clear
B)
Neurospora crassa
done
clear
C)
Mirabilis jalapa
done
clear
D)
Saccharum
done
clear
View Answer play_arrow
question_answer 122) Which of the following is an ammonifying bacterium?
A)
Clostridium
done
clear
B)
Azotobacter
done
clear
C)
Nitrosococcus
done
clear
D)
Bacillus ramosus
done
clear
View Answer play_arrow
question_answer 123) In Selaginella, normally the number of prothallial cell is
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
numerous
done
clear
View Answer play_arrow
question_answer 124) Hybrid vigour is due to
A)
heterozygosity
done
clear
B)
superiority of genes
done
clear
C)
homozygosity
done
clear
D)
mixing of cytoplasm of male and female gametes
done
clear
View Answer play_arrow
question_answer 125) Agar - agar is obtained from
A)
red algae
done
clear
B)
brown algae
done
clear
C)
cyanobacteria
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 126) Coralloid roots belong to
A)
Cycos
done
clear
B)
Pinus
done
clear
C)
Fern
done
clear
D)
Selaginella
done
clear
View Answer play_arrow
question_answer 127) An efficient root inducing chemical useful in horticulture is
A)
GAA
done
clear
B)
IBA
done
clear
C)
NAA
done
clear
D)
2, 4-D
done
clear
View Answer play_arrow
question_answer 128) Which of the following is synthesized in the dark- reaction of photosynthesis?
A)
NADPH2
done
clear
B)
ATP
done
clear
C)
Oxygen
done
clear
D)
PGA
done
clear
View Answer play_arrow
question_answer 129) The effect of pollen on the character of seed coat or pericarp is called
A)
ruminate endosperm
done
clear
B)
xenia
done
clear
C)
metaxenia
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 130) Shell of Mollusca is produced by its
A)
radulla
done
clear
B)
thorax
done
clear
C)
mantle
done
clear
D)
abdomen
done
clear
View Answer play_arrow
question_answer 131) The main function of loop of Hente is
A)
conservation of water
done
clear
B)
filtration of blood
done
clear
C)
formation of urine
done
clear
D)
filtration of urine
done
clear
View Answer play_arrow
question_answer 132) Salmon fishes are also known as
A)
cod fish
done
clear
B)
trout
done
clear
C)
cartilaginous fish
done
clear
D)
bony fish
done
clear
View Answer play_arrow
question_answer 133) In mammals, digestion of starch starts from
A)
mouth
done
clear
B)
stomach
done
clear
C)
oesophagus
done
clear
D)
duodenum
done
clear
View Answer play_arrow
question_answer 134) Green muffler is used against, which type of pollution?
A)
air
done
clear
B)
water
done
clear
C)
soil
done
clear
D)
noise
done
clear
View Answer play_arrow
question_answer 135) The joint between human skull bones is called
A)
fibrous
done
clear
B)
synovial
done
clear
C)
cartilaginous
done
clear
D)
hinge
done
clear
View Answer play_arrow
question_answer 136) Which of the following is not an amino acid?
A)
Arginine
done
clear
B)
Lysine
done
clear
C)
Thymine
done
clear
D)
Tryptophan
done
clear
View Answer play_arrow
question_answer 137) The extra embryonic membranes of mammalian embryo are derived from
A)
trophoblast
done
clear
B)
formative cells
done
clear
C)
follicle cells
done
clear
D)
inner cell mass
done
clear
View Answer play_arrow
question_answer 138) Any plant susceptible to any disease is commonly known as
A)
tolerant host
done
clear
B)
inoculation
done
clear
C)
host
done
clear
D)
colonization
done
clear
View Answer play_arrow
question_answer 139) Minimata disease was caused due to pollution of water with
A)
AgNO3
done
clear
B)
oil
done
clear
C)
mercuric chloride
done
clear
D)
DDT
done
clear
View Answer play_arrow
question_answer 140) Aggregate fruit develops from
A)
syncarpous ovary
done
clear
B)
multicarpellary, syncarpous ovary
done
clear
C)
unilocular ovary
done
clear
D)
multicarpellary, apocarpous ovary
done
clear
View Answer play_arrow
question_answer 141) Which of the following is a sex-linked character?
A)
Polio
done
clear
B)
Diabetes
done
clear
C)
Baldness
done
clear
D)
Colorblindness
done
clear
View Answer play_arrow
question_answer 142) Urinary bladder is absent in
A)
reptiles
done
clear
B)
aves
done
clear
C)
amphibians
done
clear
D)
mammals
done
clear
View Answer play_arrow
question_answer 143) Which of the following disease occurs only in males?
A)
Fabrys disease
done
clear
B)
Gauchers disease
done
clear
C)
Lesch- Nyhan disease
done
clear
D)
Hunters disease
done
clear
View Answer play_arrow
question_answer 144) The Taj Mahal is threatened due to effect of
A)
chlorine
done
clear
B)
hydrogen
done
clear
C)
sulphur dioxide
done
clear
D)
oxygen
done
clear
View Answer play_arrow
question_answer 145) Haploid endosperm is found in
A)
wheat
done
clear
B)
gram
done
clear
C)
gymnosperm
done
clear
D)
algae
done
clear
View Answer play_arrow
question_answer 146) Which of the following ecosystem has the highest gross primary productivity?
A)
Rain forest
done
clear
B)
Grassland
done
clear
C)
Coral reef
done
clear
D)
Mangroves
done
clear
View Answer play_arrow
question_answer 147) In double fertilization, second male gamete and secondary nucleus fuse to form
A)
embryo
done
clear
B)
embryo sac
done
clear
C)
endosperm
done
clear
D)
gamete
done
clear
View Answer play_arrow
question_answer 148) The biofertilizers are
A)
cowdung, manure
done
clear
B)
Anabaena and Azolla
done
clear
C)
chemical manure
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 149) VNTRs is used in
A)
regulation of plant growth hormones
done
clear
B)
increasing the rate of photosynthesis in plant of desert zones
done
clear
C)
DNA fingerprinting
done
clear
D)
protoplasmic culture
done
clear
View Answer play_arrow
question_answer 150) Mental retardation in man associated with sex chromosomal abnormality is usually due to
A)
reduction in X complement
done
clear
B)
increase in X complement
done
clear
C)
moderate increase in Y complement
done
clear
D)
large increase in X complement
done
clear
View Answer play_arrow
question_answer 151) What base is responsible for hot spots in spontaneous point mutation?
A)
Adenine
done
clear
B)
Guanine
done
clear
C)
5- bromouracil
done
clear
D)
5- methylcytocine
done
clear
View Answer play_arrow
question_answer 152) A fruit, developing from an inflorescence is called
A)
etaerio
done
clear
B)
composite fruit
done
clear
C)
achene
done
clear
D)
berry
done
clear
View Answer play_arrow
question_answer 153) The plants may face wilting due to use of excess fertilizers because of
A)
endosmosis
done
clear
B)
exosmosis
done
clear
C)
imbibition
done
clear
D)
transpiration
done
clear
View Answer play_arrow
question_answer 154) Banana is propagated by
A)
seeds
done
clear
B)
leaf base
done
clear
C)
suckers
done
clear
D)
stem
done
clear
View Answer play_arrow
question_answer 155) In which type of ovule, micropyle, chalaza, funicle are in one vertical plane?
A)
Orthotropous
done
clear
B)
Anatropous
done
clear
C)
Circinotropous
done
clear
D)
Amphitropous
done
clear
View Answer play_arrow
question_answer 156) Brain sand is found in
A)
pineal body
done
clear
B)
thymus gland
done
clear
C)
thyroid gland
done
clear
D)
pituitary gland
done
clear
View Answer play_arrow
question_answer 157) Thylakoids are present in
A)
nucleus
done
clear
B)
mitochondria
done
clear
C)
chloroplast
done
clear
D)
cytoplasm
done
clear
View Answer play_arrow
question_answer 158) Sexual reproduction is absent in
A)
Mango
done
clear
B)
Cycos
done
clear
C)
Pinus
done
clear
D)
E. coli
done
clear
View Answer play_arrow
question_answer 159)
Match List- I (lamina shape) with List- II (examples in which they are found) and select the correct answer using the codes given below the lists. List - I List - II A. B. C. D. Acicular Linear Lanceolate Oblong 1. 2. 3. 4. Grass Nerium Banana Pine
A)
A B C D 4 1 2 3
done
clear
B)
4 1 3 2
done
clear
C)
4 2 3 1
done
clear
D)
4 3 2 1
done
clear
View Answer play_arrow
question_answer 160) Monocarpic plant is
A)
neem
done
clear
B)
bamboo
done
clear
C)
apple
done
clear
D)
mango
done
clear
View Answer play_arrow
question_answer 161) Sporangia bearing leaf is called
A)
cataphyll
done
clear
B)
sporophyll
done
clear
C)
leaflet
done
clear
D)
cladophyll
done
clear
View Answer play_arrow
question_answer 162) BOD stands for
A)
biological oxide demand
done
clear
B)
biological oxygen deduction
done
clear
C)
basic oxygen demand
done
clear
D)
biological oxygen demand
done
clear
View Answer play_arrow
question_answer 163) The book Origin of Species is written by
A)
Aristotle
done
clear
B)
Darwin
done
clear
C)
Watson
done
clear
D)
Lamarck
done
clear
View Answer play_arrow
question_answer 164) Product of photosynthesis in blue-green algae is
A)
glycogen like
done
clear
B)
glucoside
done
clear
C)
globulin
done
clear
D)
glycerophosphate
done
clear
View Answer play_arrow
question_answer 165) Pasteur used the term for fermentation
A)
zymogenosis
done
clear
B)
zymosis
done
clear
C)
anaerobic respiration
done
clear
D)
combustion
done
clear
View Answer play_arrow
question_answer 166) The first dicarboxylic acid in Krebs cycle is
A)
isocitric acid
done
clear
B)
pyruvic acid
done
clear
C)
oxalo acetic acid
done
clear
D)
\[\alpha \]- ketoglutaric acid
done
clear
View Answer play_arrow
question_answer 167) Chromosomes with median centromere and equal arms are termed
A)
telocentric
done
clear
B)
acentric
done
clear
C)
metacentric
done
clear
D)
submetacentric
done
clear
View Answer play_arrow
question_answer 168) Husk fibre coir of commerce is obtained from which part of Cocos nucifera?
A)
Epicarp
done
clear
B)
Endocarp
done
clear
C)
Mesocarci
done
clear
D)
Seed coat
done
clear
View Answer play_arrow
question_answer 169) The brood pouch in sea horse is found at the belly of
A)
male
done
clear
B)
female
done
clear
C)
absent
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 170) Flying fish is
A)
Scoliodon
done
clear
B)
Hippocampus
done
clear
C)
Exocetus
done
clear
D)
Torpedo
done
clear
View Answer play_arrow
question_answer 171) Turtle or tortoise are the members of
A)
Pisces
done
clear
B)
Amphibia
done
clear
C)
Reptilia
done
clear
D)
Mammalia
done
clear
View Answer play_arrow
question_answer 172) Mammalian character is
A)
seven cervical vertebrae
done
clear
B)
pulmonary respiration
done
clear
C)
sebaceous glands
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 173) Which one of the following will be different in different animals?
A)
Carbohydrates
done
clear
B)
lipids
done
clear
C)
Proteins
done
clear
D)
Vitamins
done
clear
View Answer play_arrow
question_answer 174) Mutualism is
A)
negative relationship
done
clear
B)
positive relationship
done
clear
C)
non- effective relationship
done
clear
D)
harmful relationship
done
clear
View Answer play_arrow
question_answer 175) What type of plants will flower when short photoperiods are followed by long photoperiods?
A)
Long- short day plants
done
clear
B)
Short- long day plants
done
clear
C)
Intermediate plants
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 176) The bacteria breaking complex inorganic compounds in simple form are called
A)
parasites
done
clear
B)
scavengers
done
clear
C)
transformers
done
clear
D)
inducers
done
clear
View Answer play_arrow
question_answer 177) Carboxy haemoglobin is formed by combining haemoglobin with
A)
\[{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 178) Pancoasts syndrome is associated with
A)
neoplasm
done
clear
B)
neophrania
done
clear
C)
neophilism
done
clear
D)
neophobia
done
clear
View Answer play_arrow
question_answer 179) Nuhns glands are related with
A)
tongue
done
clear
B)
ear
done
clear
C)
hair
done
clear
D)
nose
done
clear
View Answer play_arrow
question_answer 180) When the Respiratory Quotient (RQ) is greater than 1, it indicates
A)
only aerobic respiration is taking place
done
clear
B)
only anaerobic respiration is taking place
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 181) Dark purplish organ lying on the left side of abdomen is called
A)
liver
done
clear
B)
spleen
done
clear
C)
gallbladder
done
clear
D)
appendix
done
clear
View Answer play_arrow
question_answer 182) An octamer of four histones complexed with DNA is called
A)
endosome
done
clear
B)
puff ring
done
clear
C)
nucleotine
done
clear
D)
nucleosome
done
clear
View Answer play_arrow
question_answer 183) The leaves of Mimosa pudica when touched, droop down due to
A)
seismonasty
done
clear
B)
epinasty
done
clear
C)
nyctinasty
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 184) Corpora quadrigemina is related with
A)
vision
done
clear
B)
smell
done
clear
C)
taste
done
clear
D)
touch
done
clear
View Answer play_arrow
question_answer 185) Cryopreservation refers to germplasm protection by
A)
breeding with wild varieties
done
clear
B)
energy flow through each trophic level
done
clear
C)
high temperature treatment
done
clear
D)
low temperature treatment
done
clear
View Answer play_arrow
question_answer 186) Pheromone is
A)
produced by the environment
done
clear
B)
produced by the body and released outside
done
clear
C)
taken from outside into the body
done
clear
D)
it is not a hormone but a pigment
done
clear
View Answer play_arrow
question_answer 187) The process of maturation of reproductive cells in the testes is known as
A)
spermatocyte
done
clear
B)
spermatogenesis
done
clear
C)
androgenesis
done
clear
D)
spermiogenesis
done
clear
View Answer play_arrow
question_answer 188) The branch of science dealing with senescence is called
A)
Serology
done
clear
B)
Genology
done
clear
C)
Gerontology
done
clear
D)
Cosmology
done
clear
View Answer play_arrow
question_answer 189) Foolish seedling disease is associated with the discovery of
A)
auxin
done
clear
B)
gibberellin
done
clear
C)
cytokinin
done
clear
D)
abscisic acid
done
clear
View Answer play_arrow
question_answer 190) Female Anopheles is
A)
endogenous host for Plasmodium
done
clear
B)
exogenous host for Plasmodium
done
clear
C)
initial host
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 191) Ultrastructure of gene includes
A)
cistron
done
clear
B)
muton
done
clear
C)
recon
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 192) Family- Leguminosae is divided on the basis of
A)
gynoecium
done
clear
B)
corolla
done
clear
C)
aestivation of calyx and corolla
done
clear
D)
corolla and androecium
done
clear
View Answer play_arrow
question_answer 193) Which of the following is communicable disease?
A)
Influenza
done
clear
B)
Diabetes
done
clear
C)
Hypertension
done
clear
D)
Kwoshiorkor
done
clear
View Answer play_arrow
question_answer 194) Y-chromosome of Drosophila contains genes for
A)
maleness
done
clear
B)
femaleness
done
clear
C)
infertility
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 195) Interferons were discovered by
A)
Gajdusek
done
clear
B)
Stanley and Prusiner
done
clear
C)
Isaacs and Lindemann
done
clear
D)
Randies
done
clear
View Answer play_arrow
question_answer 196) Which of the following is a living fossil?
A)
Nautilus
done
clear
B)
Peripatus
done
clear
C)
Limulus
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 197) Sudden reappearance of some ancestral feature is
A)
atavism
done
clear
B)
segregation
done
clear
C)
epistasis
done
clear
D)
hypostasis
done
clear
View Answer play_arrow
question_answer 198) Which of the following is a pesticide?
A)
DDT
done
clear
B)
BHC
done
clear
C)
Aldrin
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 199) Glycolate cycle is meant for the metabolism a
A)
carbohydrates
done
clear
B)
amino acids
done
clear
C)
proteins
done
clear
D)
lipids
done
clear
View Answer play_arrow
question_answer 200) Cheese is prepared by using enzyme
A)
trypsin
done
clear
B)
rennin
done
clear
C)
zymase
done
clear
D)
dextran
done
clear
View Answer play_arrow
 9uF luF 4.5 |af 6^F
9uF luF 4.5 |af 6^F