question_answer 1) A 10 \[\mu F\]capacitor is charged by a battery of emf 200V. The energy drawn from the battery, and the energy stored in the capacitor, are respectively
A)
0.05 J and 0 m J
done
clear
B)
0.05 mJ and 0.05 m J
done
clear
C)
0.4 J and 0.2 J
done
clear
D)
1.0 m J and 0.5 m J
done
clear
View Answer play_arrow
question_answer 2) A light spiral spring supports a 200 g weight at its lower end. It oscillates up and down with a period of 1 s. How much weight (gram) must be removed from the lower end to reduce the period to 0.5 s ?
A)
200
done
clear
B)
50
done
clear
C)
53
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 3) A source of sound of frequency n and a listener approach each other with a velocity equal to \[\frac{1}{20}\] of velocity of sound. The apparent frequency heard by the listener is
A)
\[\left( \frac{21}{19} \right)n\]
done
clear
B)
\[\left( \frac{20}{21} \right)n\]
done
clear
C)
\[\left( \frac{21}{20} \right)n\]
done
clear
D)
\[\left( \frac{19}{20} \right)n\]
done
clear
View Answer play_arrow
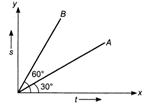
question_answer 4)
The displacement-time graphs of two bodies and B are shown in figure. The ratio \[\left( \frac{{{v}_{A}}}{{{v}_{B}}} \right)n\] of velocity of A to velocity of B, is
A)
\[\frac{1}{\sqrt{3}}\]
done
clear
B)
\[\sqrt{3}\]
done
clear
C)
\[\frac{1}{3}\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 5) A car is moving in a circular path of radius 500 m wih a speed of 30 m/s. If the speed is increasing, at the rate of \[\text{2 m}/{{\text{s}}^{\text{2}}},\] the resultant acceleration will be
A)
\[\text{2 m}/{{\text{s}}^{\text{2}}}\]
done
clear
B)
\[\text{2}.\text{5 m}/{{\text{s}}^{\text{2}}}\]
done
clear
C)
\[\text{2}.\text{7 m}/{{\text{s}}^{\text{2}}}\]
done
clear
D)
\[\text{4 m}/{{\text{s}}^{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 6) An AC supply of 100 V is applied to a capacitor of capacitance 20 \[\mu \text{F}.\]If the current in the circuit is 0.628 A, the frequency of AC. must be
A)
50 Hz
done
clear
B)
60 Hz
done
clear
C)
25 Hz
done
clear
D)
40 Hz
done
clear
View Answer play_arrow
question_answer 7) A cell of bmfX is connected across a resistor R. The potential difference across the wire is measured as \[Y.\]The internal resistance of the cell should be
A)
\[\frac{X-Y}{R}\]
done
clear
B)
\[\frac{(X-Y)R}{Y}\]
done
clear
C)
\[\frac{(X-Y)Y}{R}\]
done
clear
D)
\[(X-Y)R\]
done
clear
View Answer play_arrow
question_answer 8) Which of the following group is diamagnetic?
A)
Copper, hydrogen, silver
done
clear
B)
Copper, hydrogen, argon
done
clear
C)
Hydrogen, oxygen, argon
done
clear
D)
Copper, silver, oxygen
done
clear
View Answer play_arrow
question_answer 9) A wire has frequency \[f.\] Its length is doubled by stretching. Its frequency now will be
A)
1.4\[f\]
done
clear
B)
0.7\[f\]
done
clear
C)
2\[f\]
done
clear
D)
\[f\]
done
clear
View Answer play_arrow
question_answer 10) If a drop of water is introduced between the glass plate and a convex lens in a Newtons ring system, the ring system
A)
expands
done
clear
B)
contracts
done
clear
C)
remains same
done
clear
D)
first expands then contracts
done
clear
View Answer play_arrow
question_answer 11) A nicol prism is based on the action of
A)
double refraction
done
clear
B)
refraction
done
clear
C)
dichroism
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 12) A source of light suspended above a circular table at a height equal to the radius of the table gives an intensity I at the centre of the table, the intensity at the edge of the table would be (Assuming illuminace remains the same)
A)
0.251
done
clear
B)
0.51
done
clear
C)
0.77
done
clear
D)
2.87
done
clear
View Answer play_arrow
question_answer 13) The boiling point of mercury is \[367{}^\circ C\]. A mercury thermometer can be used to measure a temperature of \[500{}^\circ C\]
A)
by filling the space above mercury with oxygen at high pressure
done
clear
B)
by filling the space above mercury with nitrogen at low pressure
done
clear
C)
by filling the space above mercury with nitrogen at high pressure
done
clear
D)
by keeping the space above mercury as vacuum
done
clear
View Answer play_arrow
question_answer 14) 2 moles of monoatomic gas is mixed with 1 mole of a diatomic gas. Then y for the mixture is
A)
1.4
done
clear
B)
1.55
done
clear
C)
1.62
done
clear
D)
1.67
done
clear
View Answer play_arrow
question_answer 15) A body of mass 2 kg has an initial velocity of \[\text{5 m}{{\text{s}}^{-1}}\] along OE and it is subjected to a force of 4 N in a direction perpendicular to \[\text{OE}.\]The distance of the body from 0 after 4 s will be
A)
20m
done
clear
B)
48m
done
clear
C)
26m
done
clear
D)
12m
done
clear
View Answer play_arrow
question_answer 16) A solid cylinder of radius r rolls down on an inclined plane without slipping. The speed of the centre of mass when it reaches the bottom, is
A)
\[\sqrt{3gh}\]
done
clear
B)
\[\sqrt{4g}\]
done
clear
C)
\[\sqrt{\frac{3gh}{4}}\]
done
clear
D)
\[\sqrt{\frac{4gh}{3}}\]
done
clear
View Answer play_arrow
question_answer 17) The ratio of the radii of two spheres of the same mass, having the same moment of inertia about their diameters, one hollow and other solid, is
A)
\[\sqrt{3}:\sqrt{5}\]
done
clear
B)
\[\sqrt{4g}\]
done
clear
C)
25 9
done
clear
D)
9 25
done
clear
View Answer play_arrow
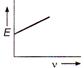
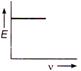
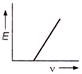
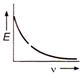
question_answer 18) The correct graph representing the relation between energy (E) of photoelectrons and frequency v of incident light is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 19) Nuclear force is
A)
short range and charge dependent
done
clear
B)
short range and charge independent
done
clear
C)
long range and charge dependent
done
clear
D)
long range and charge independent
done
clear
View Answer play_arrow
question_answer 20) The angular velocity of earth at present is \[\omega \] With what angular velocity should it rotate \[\omega \] that weight of a body at the equator appears\[\omega \] be zero?
A)
289\[\omega \]
done
clear
B)
17\[\omega \]
done
clear
C)
8\[\omega \]
done
clear
D)
2\[\omega \]
done
clear
View Answer play_arrow
question_answer 21) A force \[\overset{\to }{\mathop{F}}\,~=\text{ 6}\overset{\hat{\ }}{\mathop{\text{i}}}\,\text{ }+\text{ 5}\overset{\hat{\ }}{\mathop{\text{J}}}\,\]newton acts and displaces the body through \[\overset{\to }{\mathop{s}}\,~=\text{ 3}\overset{\hat{\ }}{\mathop{\text{i}}}\,\text{ }+\text{ 2}\overset{\hat{\ }}{\mathop{k}}\,\]metre. Then the work done is given by
A)
8J
done
clear
B)
28 J
done
clear
C)
18 J
done
clear
D)
10 J
done
clear
View Answer play_arrow
question_answer 22) The radius of aluminium nucleus (in fermi nearly
A)
1.5
done
clear
B)
2.5
done
clear
C)
4.5
done
clear
D)
13.5
done
clear
View Answer play_arrow
question_answer 23) A triode having \[{{\text{r}}_{p}}\text{ }=\text{ 25 k}\Omega \]is used as an oscillator with a load of 50 k\[\Omega \]. If gain is 20, amplification factor is
A)
75
done
clear
B)
50
done
clear
C)
30
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 24) The neutron has
A)
electron charge and spin
done
clear
B)
no charge but has spin
done
clear
C)
no charge and no spin
done
clear
D)
no charge but has exact mass of proton
done
clear
View Answer play_arrow
question_answer 25) When a hydrogen bomb explodes, which of the following is used ?
A)
Fission
done
clear
B)
Fusion
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 26) A hollow metal ball 8 cm in diameter is given a charge \[-4\times {{10}^{-8}}C.\] The potential on the surface of the ball is
A)
zero
done
clear
B)
-90V
done
clear
C)
-9000V
done
clear
D)
-900V
done
clear
View Answer play_arrow
question_answer 27) n identical resistors each of resistance r when connected in parallel, have a total resistance R. When these resistors are connected in series, then effective resistance in terms ofR is
A)
\[\frac{R}{{{n}^{2}}}\]
done
clear
B)
\[{{n}^{2}}R\]
done
clear
C)
\[n\,R\]
done
clear
D)
\[\frac{R}{n\,}\]
done
clear
View Answer play_arrow
question_answer 28) Two electric bulbs, one of 200 V, 40 W and the other of 200V, 100W are connected in a domestic circuit. Then
A)
the resistance of the bulb of 100 W is more
done
clear
B)
the resistance of the bulb of 40 W is more
done
clear
C)
the resistance of both the bulbs are same
done
clear
D)
they have equal currents through them
done
clear
View Answer play_arrow
question_answer 29) Two identical capacitors are joined in parallel and charged to a potential V. Then these are separated and connected in series with the positive plate of one connected to the negative of the other. Then the
A)
potential difference between the plates becomes 2V
done
clear
B)
energy stored in the system increases
done
clear
C)
charges on the free plates are enhanced
done
clear
D)
charges on the plates connected together are destroyed
done
clear
View Answer play_arrow
question_answer 30) \[\left[ M{{L}^{2}}{{T}^{-3}}{{I}^{-1}} \right]\]is the dimension of
A)
electric field
done
clear
B)
capacity
done
clear
C)
potential
done
clear
D)
permittivity
done
clear
View Answer play_arrow
question_answer 31) The speed of the sound in air is 333 m/s. The fundamental frequency of the open pipe is 333 Hz. The second overtone of the open organ pipe can be produced with a pipe of length
A)
0.5 m
done
clear
B)
1.0m
done
clear
C)
1.5m
done
clear
D)
2.0m
done
clear
View Answer play_arrow
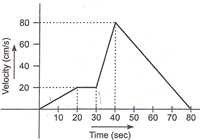
question_answer 32)
The \[v-t\]graph of a moving object is given in figure. The maximum acceleration is
A)
\[\text{1 cm}/{{\text{s}}^{\text{2}}}\]
done
clear
B)
\[\text{2 cm}/{{\text{s}}^{\text{2}}}\]
done
clear
C)
\[\text{3 cm}/{{\text{s}}^{\text{2}}}\]
done
clear
D)
\[\text{6 cm}/{{\text{s}}^{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 33) Water rises in a capillary tube up to a length 10 cm. If the tube is inclined at \[45{}^\circ \], the length of water rises in tube will be
A)
\[10\,cm\]
done
clear
B)
\[10\sqrt{2}\,\,cm\]
done
clear
C)
\[\frac{10}{\sqrt{2}}\,\,cm\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 34) As compared to a person with white skin, another person with dark (black) skin will experience
A)
less heat and more cold
done
clear
B)
more heat and more cold
done
clear
C)
more heat and less cold
done
clear
D)
less heat and less cold
done
clear
View Answer play_arrow
question_answer 35) If tube length of astronomical telescope is 105 cm and magnifying power is 20 for normal setting. The focal length of objective is
A)
100cm
done
clear
B)
10cm
done
clear
C)
20cm
done
clear
D)
25cm
done
clear
View Answer play_arrow
question_answer 36) At a certain place the horizontal component of the earths magnetic field is H and the angle of dip is 45°. The total intensity of the field at that place will be
A)
H
done
clear
B)
\[\sqrt{2}H\]
done
clear
C)
2 H
done
clear
D)
\[{{\text{H}}^{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 37) If transition temperature is doubled, the critical field will
A)
become half
done
clear
B)
become double
done
clear
C)
remain the same
done
clear
D)
become four times
done
clear
View Answer play_arrow
question_answer 38) Hydrogen and helium volume V at same temperature T and same pressure p are mixed to have same volume V. The resulting pressure of the mixture will be
A)
\[\frac{R}{2}\]
done
clear
B)
P
done
clear
C)
2P
done
clear
D)
depending on the relative mass of the gases
done
clear
View Answer play_arrow
question_answer 39) The quantity of heat required to heat 1 mole of a monoatomic gas through 1 K at constant pressure is
A)
3.5 R
done
clear
B)
2.5 R
done
clear
C)
1.5R
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 40) Light of wavelength 500 nm is used to form interference pattern in Youngs double slit experiment. A uniform glass plate of refractive index 1.5 and thickness 0.1 mm is introduced in the path of one of the interfering beams. The number of fringes which will shift the cross wire due to this is
A)
400
done
clear
B)
300
done
clear
C)
200
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 41) If a lens is moved towards the object from a distance of 20 cm to IS cm, the magnification of the image remains the same. The focal length of the lens is
A)
18.2cm
done
clear
B)
16.8cm
done
clear
C)
17.5cm
done
clear
D)
15.5cm
done
clear
View Answer play_arrow
question_answer 42) The displacement of a particle from its mean position (in metre) is given by \[\text{y }=0.\text{2 sin }\left( \text{10}\pi \text{t }+\text{ 1}.\text{5}\pi \right)\text{ cos }\left( \text{10}\pi \text{t }+\text{ l}.5\pi \right)\]The motion of the particle is
A)
periodic but not SHM
done
clear
B)
non periodic
done
clear
C)
simple harmonic motion with period 0.1 s
done
clear
D)
simple harmonic motion with period 0.2 s
done
clear
View Answer play_arrow
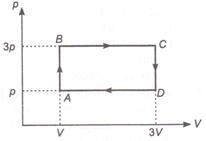
question_answer 43)
An ideal monoatomic gas is taken around the cycle ABCDA as shown in figure. The work done during the cycle is given by
A)
8pV
done
clear
B)
pV
done
clear
C)
4pV
done
clear
D)
2pV
done
clear
View Answer play_arrow
question_answer 44) When two bodies A and B are in them equilibrium
A)
the kinetic energies of all the molecules of A and B will be equal
done
clear
B)
the potential energies of all the molecules of A and B will be equal
done
clear
C)
the internal energies of the two bodies will be equal
done
clear
D)
the average kinetic energy of the molecules of two bodies will be equal
done
clear
View Answer play_arrow
question_answer 45) Two forces P and Q have a resultant perpendicular to P. The angle between forces is
A)
\[{{\tan }^{-1}}\left( -\frac{P}{Q} \right)\]
done
clear
B)
\[{{\tan }^{-1}}\left( \frac{P}{Q} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{P}{Q} \right)\]
done
clear
D)
\[{{\cos }^{-1}}\left( -\frac{P}{Q} \right)\]
done
clear
View Answer play_arrow
question_answer 46) To increase the period of a pendulum by 1C the length should be increased by percentage)
A)
21
done
clear
B)
11
done
clear
C)
10.5
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 47) A satellite is rotating around a planet in orbit of radius r with time period\[T\]if gravitational force changes according to \[{{r}^{5/2}},\] then \[{{\text{T}}^{\text{2}}}\]will be
A)
\[\propto {{r}^{3}}\]
done
clear
B)
\[\propto {{r}^{7/2}}\]
done
clear
C)
\[\propto {{r}^{9/2}}\]
done
clear
D)
\[\propto {{r}^{3/2}}\]
done
clear
View Answer play_arrow
question_answer 48) The minimum force required to move a body of mass m vertically upward is
A)
mg
done
clear
B)
mg/2
done
clear
C)
more than 2mg
done
clear
D)
more than mg
done
clear
View Answer play_arrow
question_answer 49) A body of mass 1 kg crosses a point \[o\] with a velocity \[\text{6}0\text{ m}{{\text{s}}^{-1}}.\] A force of 10 N directed towards \[o\] begins to act on it. It will again cross O in
A)
24 s
done
clear
B)
12 s
done
clear
C)
6 s
done
clear
D)
will never return to O
done
clear
View Answer play_arrow
question_answer 50) In certain region on the screen, blue and red colours fall at the same time. This region will show
A)
yellow
done
clear
B)
magenta
done
clear
C)
indigo
done
clear
D)
brown
done
clear
View Answer play_arrow
question_answer 51) A covalent chloride is
A)
\[BeC{{l}_{2}}\]
done
clear
B)
\[NaCl\]
done
clear
C)
\[MgC{{l}_{2}}\]
done
clear
D)
\[CaC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 52) An element which exhibit an oxidation state +2. How many electrons are present in its outermost shell?
A)
6
done
clear
B)
8
done
clear
C)
2
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 53) The empirical formula of a compound is CH. Its molecular weight is 78. The molecular formula of compound will be
A)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{3}}{{H}_{3}}\]
done
clear
D)
\[{{C}_{4}}{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 54) The element that is easiest to be reduced
A)
Ag
done
clear
B)
Fe
done
clear
C)
Cu
done
clear
D)
Sn
done
clear
View Answer play_arrow
question_answer 55) The molecular weight of two gases are 100 and 81 respectively. Their rates of diffusions are in the ratio
A)
81 : 100
done
clear
B)
100 : 81
done
clear
C)
10 : 9
done
clear
D)
9 : 10
done
clear
View Answer play_arrow
question_answer 56) Among \[LiCl,\,\,BeC{{l}_{2}},\,BC{{l}_{3}}\] and \[CC{{l}_{4}}\], covalent bond character follows the order
A)
\[LiCl<BeC{{l}_{2}}<BC{{l}_{3}}<CC{{l}_{4}}\]
done
clear
B)
\[LiCl<BeC{{l}_{2}}>BC{{l}_{3}}>CC{{l}_{4}}\]
done
clear
C)
\[LiCl>BeC{{l}_{2}}<BC{{l}_{3}}<CC{{l}_{4}}\]
done
clear
D)
\[LiCl>BeC{{l}_{2}}>BC{{l}_{3}}>CC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 57) The correct designation of an electron with \[n=4,\,\,l=3,\,\,m=2\] and \[s=-\frac{1}{2}\] is
A)
5s
done
clear
B)
4s
done
clear
C)
4d
done
clear
D)
4 \[f\]
done
clear
View Answer play_arrow
question_answer 58) Tritium on radioactive decay gives
A)
proton
done
clear
B)
p particle
done
clear
C)
positron
done
clear
D)
a particle
done
clear
View Answer play_arrow
question_answer 59) In two separate bulbs containing ideal gases A and B respectively, the density of gas A is twice of that of gas B while mol. wt. of gas A is half of that of gas B at the same temperature, pressure. \[\frac{{{P}_{A}}}{{{p}_{B}}}\] will be
A)
1
done
clear
B)
4
done
clear
C)
\[\frac{1}{4}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 60) On passing electric current of one ampere for 16 min and 5 s through one litre solution of\[CuC{{l}_{2}}\], all copper solution was deposited at cathode. The strength of \[CuC{{l}_{2}}\] solution was (molar mass of Cu = 63.5, faraday constant = 96500 C/mol)
A)
0.2 N
done
clear
B)
0.01 N
done
clear
C)
0.001 N
done
clear
D)
0.02 N
done
clear
View Answer play_arrow
question_answer 61) The ionic product of water...........with the increase in temperature
A)
decreases
done
clear
B)
increases
done
clear
C)
remains constant
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 62) Consider reversible reaction,\[HCN(aq){{H}^{+}}(aq)+C{{N}^{-}}(aq)\] At equilibrium the addition of \[C{{N}^{-}}(aq)\] would
A)
decrease the \[{{H}^{+}}(aq)\] ion concentration
done
clear
B)
reduce HCN (aq) concentration
done
clear
C)
increase the equilibrium constant
done
clear
D)
decrease the equilibrium constant
done
clear
View Answer play_arrow
question_answer 63) For the reaction\[C(s)+C{{O}_{2}}(g)2CO(g)\]the partial pressure for \[C{{O}_{2}}\] and CO are 4 and 8 atm respectively. \[{{K}_{p}}\] for the reaction is
A)
1 atm
done
clear
B)
5 atm
done
clear
C)
2 atm
done
clear
D)
16 atm
done
clear
View Answer play_arrow
question_answer 64) The binding energy per nucleon of oxygen atom \[_{8}{{O}^{16}}\], which has a mass 15.994910 u is : (mass of neutron = 1.008665, mass of proton = 1.007277 u, mass of electron = 0.0005486 u) is
A)
7.976 MeV
done
clear
B)
8.542 MeV
done
clear
C)
6.62 MeV
done
clear
D)
931 MeV
done
clear
View Answer play_arrow
question_answer 65) \[{{t}_{1/2}}\] for Kr is 10.6 yr. The time taken for 99.9% decay of Kr is
A)
106 yr
done
clear
B)
1060 yr
done
clear
C)
1.06 yr
done
clear
D)
10.6 yr
done
clear
View Answer play_arrow
question_answer 66) A gas is found to have formula \[{{(CO)}_{n}}\]. If its vapour density is 56, the value of n will be
A)
7
done
clear
B)
5
done
clear
C)
4
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 67) The same amount of electricity was passed through two cells containing molten \[A{{l}_{2}}{{O}_{3}}\] and molten \[NaCl\]. If 1.8 g of Al were liberated in one cell, the amount of Na liberated in other cell is
A)
2.8 g
done
clear
B)
3.2 g
done
clear
C)
4.6 g
done
clear
D)
6.8 g
done
clear
View Answer play_arrow
question_answer 68) The molal freezing point constant for water is\[{{1.86}^{o}}C/mol\]. If 342 g of sucrose \[({{C}_{12}}{{H}_{22}}{{O}_{11}})\]is dissolved in 1000 g of water, the solution will freeze at
A)
\[+{{1.86}^{o}}C\]
done
clear
B)
\[-{{1.86}^{o}}C\]
done
clear
C)
\[{{2.42}^{o}}C\]
done
clear
D)
\[{{3.92}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 69) pH of 0.005 \[{{H}_{2}}S{{O}_{4}}\] solution is
A)
2
done
clear
B)
3
done
clear
C)
2.301
done
clear
D)
3.6990
done
clear
View Answer play_arrow
question_answer 70) The molal elevation constant is the ratio of elevation in b.p. to
A)
molality
done
clear
B)
mole fraction
done
clear
C)
molarity
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 71) Which of the following is the strongest acid?
A)
\[HCl{{O}_{4}}\]
done
clear
B)
\[HI{{O}_{3}}\]
done
clear
C)
\[HN{{O}_{3}}\]
done
clear
D)
\[HBr{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 72) For spontaneous process
A)
G decreases
done
clear
B)
G increases
done
clear
C)
S decreases
done
clear
D)
S = 0
done
clear
View Answer play_arrow
question_answer 73) If \[C(s)+{{O}_{2}}(g)\,\,\xrightarrow{{}}\,\,C{{O}_{2}}(g)\,;\] \[\Delta H=R\] and\[CO(g)+\frac{1}{2}{{O}_{2}}(g)\,\,\,\xrightarrow{{}}\,\,C{{O}_{2}}(g);\]\[\Delta H=S\] then heat of formation of CO is
A)
R + S
done
clear
B)
R - S
done
clear
C)
\[R\times S\]
done
clear
D)
S - R
done
clear
View Answer play_arrow
question_answer 74) When \[NaOH\] is added to a solution of\[C{{H}_{3}}COONa\], then
A)
the cone. of both \[O{{H}^{-}}\] and \[C{{H}_{3}}CO{{O}^{-}}\]increases
done
clear
B)
the cone. of \[C{{H}_{3}}CO{{O}^{-}}\] decreases
done
clear
C)
the cone. of \[C{{H}_{3}}CO{{O}^{-}}\] increases
done
clear
D)
dissociation of \[C{{H}_{3}}COONa\] increases
done
clear
View Answer play_arrow
question_answer 75) Chile salt petre is
A)
\[CaC{{O}_{3}}\]
done
clear
B)
\[NaN{{O}_{3}}\]
done
clear
C)
\[MgC{{O}_{3}}\]
done
clear
D)
\[KN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 76) Under what conditions will a pure sample of ideal gas not only exhibit a pressure of 1 atm, but also a concentration of 2 mol per litre?
A)
At STP
done
clear
B)
When volume is 22.4 L
done
clear
C)
At 6.1 K
done
clear
D)
When R has no unit
done
clear
View Answer play_arrow
question_answer 77) For the gas phase reaction,\[{{C}_{2}}{{H}_{4}}+{{H}_{2}}{{C}_{2}}{{H}_{6}}\]\[(\Delta H=-32.7\,\,kcal)\]carried out in a vessel, the equilibrium cone. of \[{{C}_{2}}{{H}_{6}}\] can be increased by
A)
increasing the pressure
done
clear
B)
decreasing the temperature
done
clear
C)
adding some Hz
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 78) Pick out the isoelectronic structure from the following\[(I)\overset{+}{\mathop{C}}\,{{H}_{3}}(II){{H}_{3}}\overset{+}{\mathop{O}}\,(III)N{{H}_{3}}\,\,(IV)\overset{-}{\mathop{C}}\,{{H}_{3}}\]
A)
I and II
done
clear
B)
III and IV
done
clear
C)
II, III and IV
done
clear
D)
III and IV and I
done
clear
View Answer play_arrow
question_answer 79) Mg on heating to redness in an atmosphere of \[{{N}_{2}}\] and then on treating with \[{{H}_{2}}O\] gives
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{N}_{2}}\]
done
clear
C)
\[P{{H}_{3}}\]
done
clear
D)
MgO
done
clear
View Answer play_arrow
question_answer 80) Bond energy is highest for
A)
Ge?Ge
done
clear
B)
C?C
done
clear
C)
Sn?Sn
done
clear
D)
Si?Si
done
clear
View Answer play_arrow
question_answer 81) Which acid derivative on hydrolysis, will give brown precipitate with Nesslefs reagent?
A)
Acid anhydride
done
clear
B)
Acid amide
done
clear
C)
Acid chloride
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 82) n-hexadecane (cetane) has cetane number
A)
110
done
clear
B)
90
done
clear
C)
100
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 83) Alkyne occur in nature in
A)
partially free state
done
clear
B)
not in free state
done
clear
C)
free state
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 84) Alkaline earth metals are
A)
reducing agent
done
clear
B)
amphoteric
done
clear
C)
dehydrating agent
done
clear
D)
oxidising agent
done
clear
View Answer play_arrow
question_answer 85) Copper sulphate is commercially made from copper scrap by
A)
heating with sodium sulphate
done
clear
B)
heating with sulphur
done
clear
C)
action of dil sulphuric acid and air
done
clear
D)
dissolving in hot concentrated sulphuric acid
done
clear
View Answer play_arrow
question_answer 86) To a piece of a charcoal sulphuric acid is added. Then
A)
there is no reaction
done
clear
B)
water gas is formed
done
clear
C)
\[S{{O}_{2}}\] and \[C{{O}_{2}}\] are evolved
done
clear
D)
CO and \[S{{O}_{2}}\] are evolved
done
clear
View Answer play_arrow
question_answer 87) What happens when carbonates, of IA group elements are heated?
A)
\[C{{O}_{2}}\] is given out
done
clear
B)
Water vapours are given out
done
clear
C)
Carbon dioxide and water vapours are evolved
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 88) pH of aqueous of sodium borate is
A)
>7
done
clear
B)
6 > 7
done
clear
C)
7
done
clear
D)
< 7
done
clear
View Answer play_arrow
question_answer 89) Which of the following cannot act as reducing agent?
A)
\[N{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[Cl{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 90) The major product of dehydration of n-butyl alcohol is
A)
1-butene
done
clear
B)
1-butyne
done
clear
C)
2-butene
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 91) Colourless ion among the following is
A)
\[Z{{n}^{2+}}\]
done
clear
B)
\[C{{r}^{3+}}\]
done
clear
C)
\[{{V}^{2+}}\]
done
clear
D)
\[T{{i}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 92) \[P{{I}_{3}}\] upon hydrolysis give
A)
monobasic acid and dibasic acid
done
clear
B)
monobasic and tribasic acid
done
clear
C)
monobasic acid and a salt
done
clear
D)
dibasic acid and tribasic acid
done
clear
View Answer play_arrow
question_answer 93) 1-propanol can be easily obtained from propene \[C{{H}_{3}}-CH=C{{H}_{2}}\], by oxidation in the presence of
A)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 94) Benzoic acid with \[Ba{{(OH)}_{2}}\] gives
A)
barium benzoate
done
clear
B)
benzaldehyde
done
clear
C)
benzene
done
clear
D)
toluene
done
clear
View Answer play_arrow
question_answer 95) Diazonium salt is obtained when aniline reacts with
A)
cold NaOH
done
clear
B)
\[NaN{{O}_{2}}\] and \[HCl\,(0-{{5}^{o}}C)\]
done
clear
C)
\[SnC{{l}_{2}}\] at \[{{10}^{o}}C\]
done
clear
D)
\[{{N}_{2}}O\] at \[(0-{{5}^{o}}C)\]
done
clear
View Answer play_arrow
question_answer 96) Which of the following is formed on reaction of acetaldehyde with HCN followed by hydrolysis?
A)
Butyl alcohol
done
clear
B)
Acetic acid
done
clear
C)
Oxalic acid
done
clear
D)
Lactic acid
done
clear
View Answer play_arrow
question_answer 97) Gem dihalides on treatment with alcoholic KOH give
A)
alkyne
done
clear
B)
alkene
done
clear
C)
alkane
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 98) IUPAC name of \[{{({{C}_{2}}{{H}_{5}}CO)}_{2}}O\]
A)
acetic acid
done
clear
B)
propanoic anhydride
done
clear
C)
butanone
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 99) Which of the following does not occur in free state?
A)
Pt
done
clear
B)
Os
done
clear
C)
Au
done
clear
D)
Cu
done
clear
View Answer play_arrow
question_answer 100) The number of \[\pi \] and n bonds in but-l-ene-3-yne are
A)
\[7\sigma \] and \[3\pi \]
done
clear
B)
\[8\sigma \] and \[2\pi \]
done
clear
C)
\[8\sigma \] and \[4\pi \]
done
clear
D)
\[5\sigma \] and \[5\pi \]
done
clear
View Answer play_arrow
question_answer 101) Virion is a
A)
bacterium
done
clear
B)
blue -green algae
done
clear
C)
simple virus particle
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 102) The number of stomata present per \[c{{m}^{2}}\]of a leaf is
A)
1000
done
clear
B)
less than 100
done
clear
C)
one million
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 103) Binomial nomenclature means that every organism has
A)
two names one scientific and other popular
done
clear
B)
one scientific name consisting of a generic and a specific epithet
done
clear
C)
one name give by two scientists
done
clear
D)
two names, one latinize and other of the person
done
clear
View Answer play_arrow
question_answer 104) Mesophyll is well differentiated into palisade and spongy tissue in
A)
dicot leaves
done
clear
B)
monocot leaves
done
clear
C)
xerophytic stem
done
clear
D)
hydrophytic stem
done
clear
View Answer play_arrow
question_answer 105) Cell organelle common in Monera and Protista
A)
lysosome
done
clear
B)
chloroplast
done
clear
C)
ribosome
done
clear
D)
vacuole
done
clear
View Answer play_arrow
question_answer 106) Cell wall present in water conducting tissues, represented by swollen nodules is known as
A)
tertiary wall
done
clear
B)
middle lamella
done
clear
C)
plasmalemma
done
clear
D)
primary cell wall
done
clear
View Answer play_arrow
question_answer 107) Grana is ill developed or absent in the chloroplast in the
A)
stem of Hydrilla
done
clear
B)
leaf of sunflower
done
clear
C)
bundle sheath of sugarcane leaf
done
clear
D)
mesophyll of grasses
done
clear
View Answer play_arrow
question_answer 108) A straight fertilizer is the one, which is
A)
absorbed by roots directly
done
clear
B)
absorbed by the plants from aerial spray
done
clear
C)
having only one primary nutrient
done
clear
D)
not easily leached
done
clear
View Answer play_arrow
question_answer 109) Pollenkit material is secreted by
A)
tapetum
done
clear
B)
endothecium
done
clear
C)
epidermis
done
clear
D)
endodermis
done
clear
View Answer play_arrow
question_answer 110) In certain plant species red flower colour is incompletely dominant to white flower colour (the heterozygote is pink) and tall stems are completely dominant to dwarf stem. If a tall pink plant (TtRr) is crossed with a tall white plant (TTrr), which one of the following type of plants would be produced in the offsprings?
A)
Tall pink and tall white
done
clear
B)
Dwarf pink and tall red
done
clear
C)
Dwarf red and tall pink
done
clear
D)
Tall pink and dwarf white
done
clear
View Answer play_arrow
question_answer 111) Growth rings are absent or not sharply demarcated in the trees of
A)
temperate deciduous
done
clear
B)
tropical evergreen
done
clear
C)
temperate evergreen
done
clear
D)
tropical deciduous
done
clear
View Answer play_arrow
question_answer 112) An example of naturally occurring parthenocarpic fruit is
A)
apple
done
clear
B)
mango
done
clear
C)
banana
done
clear
D)
guava
done
clear
View Answer play_arrow
question_answer 113) Water stomata are found in
A)
plants lacking normal stomata
done
clear
B)
plants inhabiting dry regions
done
clear
C)
plants inhabiting humid region
done
clear
D)
all plants
done
clear
View Answer play_arrow
question_answer 114) Polygenic concept of inheritance was first experimentally shown by
A)
Malthus (1828)
done
clear
B)
Gabon (1883)
done
clear
C)
Nilson Ehle
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 115) If a starving plant is provided with glucose, the rate of respiration would
A)
first rise than fall
done
clear
B)
become constant
done
clear
C)
decrease
done
clear
D)
increase
done
clear
View Answer play_arrow
question_answer 116) Meiosis in Dryopteris takes place at the time of
A)
gamete formation
done
clear
B)
spore formation
done
clear
C)
formation of sex organs
done
clear
D)
endosperm formation
done
clear
View Answer play_arrow
question_answer 117) First seed plant appeared during which you
A)
Silurian
done
clear
B)
Devonian
done
clear
C)
Carboniferous
done
clear
D)
Cretaceous
done
clear
View Answer play_arrow
question_answer 118) Selaginella does not show
A)
protostele
done
clear
B)
microphyllous leaves
done
clear
C)
precocious germination
done
clear
D)
circinate venation
done
clear
View Answer play_arrow
question_answer 119) Calcium encrustation and larvicidal properties are present in
A)
Chara
done
clear
B)
Oscillatoria
done
clear
C)
Diatoms
done
clear
D)
Caulerapa
done
clear
View Answer play_arrow
question_answer 120) In Spirogyra
A)
filaments, in which lateral conjugation occur are homothallic
done
clear
B)
filaments, in which sclariform conjugation occur are homothallic
done
clear
C)
filaments, in which lateral conjugation occur are heterothallic
done
clear
D)
a sexual reproduction occur by zoospores
done
clear
View Answer play_arrow
question_answer 121) Formation, growth and development of a new individual from egg is known as
A)
Embryology
done
clear
B)
Genetics
done
clear
C)
Ethnobotany
done
clear
D)
Cytology
done
clear
View Answer play_arrow
question_answer 122) Which type of moss is Funaria?
A)
Acrocarpous moss
done
clear
B)
Pleurocarpous moss
done
clear
C)
Anacrogynous moss
done
clear
D)
Cleistocarpous moss
done
clear
View Answer play_arrow
question_answer 123) Fin Funaria, the haploid structure is
A)
protonema
done
clear
B)
capsule
done
clear
C)
columella
done
clear
D)
seta
done
clear
View Answer play_arrow
question_answer 124) The lacunae in vascular bundle of monocot stem is
A)
a mucilage canal
done
clear
B)
a large sized vessel
done
clear
C)
lysigenous water cavity
done
clear
D)
metaxylem
done
clear
View Answer play_arrow
question_answer 125) Secondary phloem remains functional generally
A)
for one year
done
clear
B)
for less than one year
done
clear
C)
for many years
done
clear
D)
as long as plant is alive
done
clear
View Answer play_arrow
question_answer 126) Tubulin protein occurs in
A)
digestive enzymes
done
clear
B)
rough endoplasmic reticulum
done
clear
C)
thylakoids
done
clear
D)
microtubules
done
clear
View Answer play_arrow
question_answer 127) While preparing sauce, chatni and jams, these should not be heated in iron containers because
A)
acid reacts with iron to give black colour
done
clear
B)
makes edible material poisonous
done
clear
C)
takes a longer time to ripe
done
clear
D)
edible material becomes rusty
done
clear
View Answer play_arrow
question_answer 128) The lentic ecosystem includes
A)
gravitational water
done
clear
B)
standing water
done
clear
C)
rainwater
done
clear
D)
running water
done
clear
View Answer play_arrow
question_answer 129) Light reaction takes place in
A)
grana
done
clear
B)
endoplasmic reticulum
done
clear
C)
stroma
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 130) Which part of root absorbs both water and minerals?
A)
Zone of cell differentiation
done
clear
B)
Zone of cell formation
done
clear
C)
Zone of cell elongation
done
clear
D)
Terminal portion of roots
done
clear
View Answer play_arrow
question_answer 131) Pneumatophores are helpful in
A)
protein synthesis
done
clear
B)
respiration
done
clear
C)
carbohydrate metabolism
done
clear
D)
transpiration
done
clear
View Answer play_arrow
question_answer 132) The osmotic parameter determining the flow of water from one cell to another is
A)
OP
done
clear
B)
DPD
done
clear
C)
TP
done
clear
D)
Hydrostatic pressure
done
clear
View Answer play_arrow
question_answer 133) \[C{{O}_{2}}\]acceptor in \[{{C}_{4}}\]plant is
A)
PGA
done
clear
B)
Oxalo acetic add
done
clear
C)
RuDP
done
clear
D)
PEP
done
clear
View Answer play_arrow
question_answer 134) Which of the following movements is induced by injury?
A)
Aerotropism
done
clear
B)
Geotropism
done
clear
C)
Tromonasty
done
clear
D)
Traumatropism
done
clear
View Answer play_arrow
question_answer 135) In Desmodium gyrans when environmental conditions are favourable
A)
lateral leaflet remains static and middle one oscillate
done
clear
B)
middle leaflet remains static and lateral oscillate simultaneously
done
clear
C)
middle leaflet remains static and lateral oscillate alternately
done
clear
D)
all three leaves oscillate
done
clear
View Answer play_arrow
question_answer 136) Which of the following is called stinging nettle?
A)
Utricaurens
done
clear
B)
Adhatoda
done
clear
C)
Verbascum
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 137) In big cities, the major atmospheric pollutant is
A)
carbon monoxide and oxides of sulphur
done
clear
B)
hydrocarbon and hot air
done
clear
C)
pollens and marsh gas
done
clear
D)
ozone
done
clear
View Answer play_arrow
question_answer 138) Substance related with phototropism in shoot is
A)
ethanol
done
clear
B)
cytokinins
done
clear
C)
auxin
done
clear
D)
gibberellins
done
clear
View Answer play_arrow
question_answer 139) The structural genes in the lac operon serially comprise
A)
y, a and z cistrons
done
clear
B)
a, y and z cistrons
done
clear
C)
z, a and y cistrons
done
clear
D)
z, y and a cistrons
done
clear
View Answer play_arrow
question_answer 140) Phytoplanktons are
A)
insectivorous
done
clear
B)
chemotrophs
done
clear
C)
heterotrophs
done
clear
D)
autotrophs
done
clear
View Answer play_arrow
question_answer 141) Which of the following combines irreversibly with blood haemoglobin?
A)
\[S{{O}_{2}}\]
done
clear
B)
\[~{{O}_{3}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 142) Sugarcane is cultivated through
A)
stem cutting
done
clear
B)
root cutting
done
clear
C)
true seed
done
clear
D)
adventitious roots
done
clear
View Answer play_arrow
question_answer 143) What will happen if the supply of oxygen is decreased to an illuminated wheat plant?
A)
Its photosynthesis would decrease
done
clear
B)
Its respiration process would stop
done
clear
C)
All physiological process would stop
done
clear
D)
Its photosynthesis would increase
done
clear
View Answer play_arrow
question_answer 144) Assimilatory power in photosynthesis refers to
A)
\[ATP+NADP{{H}_{2}}+C{{O}_{2}}\]
done
clear
B)
\[ATP+NADP{{H}_{2}}\]
done
clear
C)
\[ATP\]
done
clear
D)
\[NADP{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 145) Richest source of protein is
A)
rice
done
clear
B)
gram
done
clear
C)
wheat
done
clear
D)
Glycine max
done
clear
View Answer play_arrow
question_answer 146) In cyanophage, the genetic material is
A)
RNA
done
clear
B)
DNA
done
clear
C)
Both (a) and (b)
done
clear
D)
protein
done
clear
View Answer play_arrow
question_answer 147) Maximum utilization of biotechnological techniques has been made in the field of
A)
industries
done
clear
B)
medicines
done
clear
C)
agriculture
done
clear
D)
biogas production
done
clear
View Answer play_arrow
question_answer 148) A small aquatic plant was put in each of the petri dishes ? X, Y and Z, containing different culture solutions. After six weeks the plant in dish X has the same number of leaves as it has previously and were all small and yellowish. Plant in dish Y had more leaves of normal size and dark green colour. Plants in dish Z had more leaves of normal size but very pale. Which of the following shows the element missing in the culture?
A)
X
Y
Z
Magnesium
Phosphorus
Nitrogen
done
clear
B)
X
Y
Z
Phosphorus
Magnesium
Nitrogen
done
clear
C)
X
Y
Z
Phosphorus
Nitrogen
Magnesium
done
clear
D)
X
Y
Z
Magnesium
Nitrogen
Phosphorus
done
clear
View Answer play_arrow
question_answer 149) Restriction enzyme was discovered by
A)
Alexander Flemming
done
clear
B)
Waksman
done
clear
C)
Berg
done
clear
D)
Smith, Nathans and Arber
done
clear
View Answer play_arrow
question_answer 150) The green colored pigment present in all autotrophs was named chlorophyll by
A)
Pelletier and Caventou
done
clear
B)
Julius Rober Mayer
done
clear
C)
Jean Senebier
done
clear
D)
Malvin Calvin
done
clear
View Answer play_arrow
question_answer 151) Cellulose is made up of
A)
branched chain of glucose molecules linked by \[\alpha \] -1, 4 glycosidic bond in straight chain and \[\alpha \] - 1, 6 glycosidic bond at the site of branching
done
clear
B)
branched chain of glucose molecules linked by \[\alpha \] - 1, 6 glycosidic bond straight chain and \[\alpha \] - 1, 4 glycosidic box at the site of branching
done
clear
C)
unbranched chain of glucose molecules linked by\[\beta \]- 1, 4 glycosidic bond
done
clear
D)
unbranched chain of glucose molecules linked by\[\alpha \]- 1, 6 glycosidic bond
done
clear
View Answer play_arrow
question_answer 152) Cell Adhesion and cell recognition occur due to biochemicals of cell membrane named
A)
lipids
done
clear
B)
proteins
done
clear
C)
glycoproteins and glycolipids
done
clear
D)
proteins and lipids
done
clear
View Answer play_arrow
question_answer 153) Sol-gel theory, for the first time given by
A)
Pantin
done
clear
B)
Hyman
done
clear
C)
Best
done
clear
D)
Mast
done
clear
View Answer play_arrow
question_answer 154) The concept of genus was proposed by
A)
John Ray
done
clear
B)
Tournefort
done
clear
C)
Hooker
done
clear
D)
Bessey
done
clear
View Answer play_arrow
question_answer 155) In Hydra, the beaded processes of sensory cells make synapsis with the process of
A)
nerve cell
done
clear
B)
epithelio muscular cell
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 156) Sperms in Ascaris are characterized by one unusual feature
A)
long form
done
clear
B)
lack of flagellum
done
clear
C)
motility
done
clear
D)
ability to induce meiosis in egg
done
clear
View Answer play_arrow
question_answer 157) Ascaris has
A)
paired testes and single ovary
done
clear
B)
paired ovary and single testes
done
clear
C)
single ovary and single testes
done
clear
D)
paired ovary and paired testes
done
clear
View Answer play_arrow
question_answer 158) Which of the following is not a characteristic of snakes?
A)
Eggs
done
clear
B)
Sternum
done
clear
C)
Scales
done
clear
D)
Kidney
done
clear
View Answer play_arrow
question_answer 159) Dudhwa national park is in
A)
Orissa
done
clear
B)
Gujarat
done
clear
C)
Uttar Pradesh
done
clear
D)
Himachal Pradesh
done
clear
View Answer play_arrow
question_answer 160) Leydig cells are concerned with
A)
ovary
done
clear
B)
seminiferous tubules
done
clear
C)
liver
done
clear
D)
pituitary
done
clear
View Answer play_arrow
question_answer 161) Scientific name of starfish is
A)
Echinus
done
clear
B)
Limulus
done
clear
C)
Echidna
done
clear
D)
Asterias
done
clear
View Answer play_arrow
question_answer 162) Pulse can be detected from the artery of
A)
wrist
done
clear
B)
thigh
done
clear
C)
diaphragm
done
clear
D)
humerus
done
clear
View Answer play_arrow
question_answer 163) Cranium is the protective covering of
A)
lungs
done
clear
B)
eye balls
done
clear
C)
brain
done
clear
D)
uterus
done
clear
View Answer play_arrow
question_answer 164) Immediate energy source for muscle contraction is
A)
ATP
done
clear
B)
ADP
done
clear
C)
glucose
done
clear
D)
lactic acid
done
clear
View Answer play_arrow
question_answer 165) Genetic engineering is employed to produce vaccines for
A)
herpes virus
done
clear
B)
hepatitis - B
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 166) Triazines are derived from
A)
uric acid
done
clear
B)
urea
done
clear
C)
ammonia
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 167) Kappa particles are
A)
protozoan parasites whose multiplication is controlled by host metabolites
done
clear
B)
viral particles capable of self perpetuation in host cytoplasm
done
clear
C)
endosymbiont representing Gram negative bacterial species
done
clear
D)
submicroscopic granules formed by the folding of naked DNA
done
clear
View Answer play_arrow
question_answer 168) According to Neo- Darwinism new species develops through
A)
mutation
done
clear
B)
hybridization
done
clear
C)
continuous variation with natural selection
done
clear
D)
mutation with natural selection
done
clear
View Answer play_arrow
question_answer 169) Amniocentesis is
A)
process to know about brain disease
done
clear
B)
process to determine disease of heart
done
clear
C)
process to determine hereditary disease in the embryo
done
clear
D)
process to determine amount of amino adds
done
clear
View Answer play_arrow
question_answer 170) Plant viruses contain
A)
DNA
done
clear
B)
RNA
done
clear
C)
Both (a) and (b)
done
clear
D)
Plasmids
done
clear
View Answer play_arrow
question_answer 171) The spindle microtubules are polar, their orientation is
A)
+ and - both ends towards the equator
done
clear
B)
+ end towards the poles
done
clear
C)
- ends towards the poles
done
clear
D)
+ and - both ends towards the poles
done
clear
View Answer play_arrow
question_answer 172) Go phase is
A)
phase after \[{{G}_{2}}\]
done
clear
B)
phase after M phase, in which daughter cell enters new cell cycle
done
clear
C)
arrest of cell cycle on the onset of differentiation
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 173) Mouth develops first in the embryo and anus is formed later. It is called
A)
protostomatic
done
clear
B)
deuterostomatic
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 174) In Leucosolenia, gametes develop from
A)
amoebocytes
done
clear
B)
archaeocytes
done
clear
C)
choanocytes
done
clear
D)
myocytes
done
clear
View Answer play_arrow
question_answer 175) The largest of cnidoblast is
A)
penetrant
done
clear
B)
streptoline
done
clear
C)
volvent
done
clear
D)
small glutiant
done
clear
View Answer play_arrow
question_answer 176) Which of the following sets of derivatives of integumentary structures characterise birds as glorified reptiles?
A)
Scales and claws
done
clear
B)
Syrinx and uropygial gland
done
clear
C)
Claws and uropygial gland
done
clear
D)
Syrinx and scales
done
clear
View Answer play_arrow
question_answer 177) Which of the following generally modifies into glands?
A)
Muscular tissue
done
clear
B)
Nervous tissue
done
clear
C)
Connective tissue
done
clear
D)
Epithelial tissue
done
clear
View Answer play_arrow
question_answer 178) Myelinated nerve fibres are white colored because of
A)
chromidial substance
done
clear
B)
neurolemma
done
clear
C)
myelin
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 179) Least peristalsis occurs in
A)
rectum
done
clear
B)
stomach
done
clear
C)
oesophagus
done
clear
D)
duodenum
done
clear
View Answer play_arrow
question_answer 180) Non- participation of male pronucleus in fertilization is
A)
androgenesis
done
clear
B)
polyandry
done
clear
C)
gynogenesis
done
clear
D)
polygyny
done
clear
View Answer play_arrow
question_answer 181) When the balloon of nitre -aortic balloon pump inflates more blood is carried to
A)
coronary artery
done
clear
B)
pulmonary trunk
done
clear
C)
hepatic portal
done
clear
D)
pulmonary arteries
done
clear
View Answer play_arrow
question_answer 182) Salamander can regenerate
A)
tail
done
clear
B)
limbs
done
clear
C)
external gills
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 183) In Omithine cycle, the enzyme arginase breaks down arginine into
A)
citrulline and ammonia
done
clear
B)
citrulline and urea
done
clear
C)
Omithine and ammonia
done
clear
D)
Omithine and urea
done
clear
View Answer play_arrow
question_answer 184) Parkinsonism, which is characterized by tremors and progressive rigidity of limbs is caused by degeneration of brain, neurons and neuro transmitters called
A)
dopamine
done
clear
B)
adrenaline
done
clear
C)
acetyl choline
done
clear
D)
aspartic acid
done
clear
View Answer play_arrow
question_answer 185) Yellow spot of eye is known for
A)
complex blood vascular system
done
clear
B)
high pigmentation
done
clear
C)
preponderance of cones
done
clear
D)
possession of abundant rods
done
clear
View Answer play_arrow
question_answer 186) Latissimus dorsi muscles are
A)
muscles of fore arm
done
clear
B)
muscles of lower jaw
done
clear
C)
muscles of chest
done
clear
D)
muscles of shoulder
done
clear
View Answer play_arrow
question_answer 187) Zygomatic process in mammals arises from
A)
mandible
done
clear
B)
maxilla
done
clear
C)
premaxilla
done
clear
D)
squamosal
done
clear
View Answer play_arrow
question_answer 188) Eunuchoidism is due to the faliure of production of
A)
FSH
done
clear
B)
testosterone
done
clear
C)
ICSH
done
clear
D)
estrogen
done
clear
View Answer play_arrow
question_answer 189) Which of the following stimulates production of gastric juice?
A)
Enterogasteron
done
clear
B)
Secretin
done
clear
C)
Gastrin
done
clear
D)
Enterokinase
done
clear
View Answer play_arrow
question_answer 190) First milk produced after child birth is called
A)
sebum
done
clear
B)
cerumen
done
clear
C)
true milk
done
clear
D)
colostrums
done
clear
View Answer play_arrow
question_answer 191) Embryologist can draw the fate maps of fur-I organs of embryo in
A)
blastula
done
clear
B)
morula
done
clear
C)
early gastrula
done
clear
D)
late gastrula
done
clear
View Answer play_arrow
question_answer 192) Blastopore is found in
A)
blastula and is opening of archenteror
done
clear
B)
blastula and is opening of blastocoel
done
clear
C)
gastrula and is opening of archenteroc
done
clear
D)
gastrula and is opening of blastocoels
done
clear
View Answer play_arrow
question_answer 193) Alleles of different genes that on the same chromosome may occasionally be separated by a phenomenon known as
A)
pleiotropy
done
clear
B)
epistatis
done
clear
C)
continuous variation
done
clear
D)
crossing over
done
clear
View Answer play_arrow
question_answer 194) Gene with multiple effect is
A)
supplementary
done
clear
B)
pleiotropic
done
clear
C)
epistatic
done
clear
D)
codominant
done
clear
View Answer play_arrow
question_answer 195) Name of first one toed horse is
A)
Mesohippus
done
clear
B)
Eohippus
done
clear
C)
Pliohippus
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 196) For evolution most important requirement is
A)
continuity of germplasm
done
clear
B)
variation
done
clear
C)
adaptation of acquired traits
done
clear
D)
natural selection
done
clear
View Answer play_arrow
question_answer 197) The part of Papaver soniniferum, from which opium is obtained
A)
seed
done
clear
B)
stem and leaf
done
clear
C)
unripe fruits
done
clear
D)
mature fruits
done
clear
View Answer play_arrow
question_answer 198) Transgenic crops are modified through genetic engineering to develop natural resistance to insect pests. Which one is a transgenic plant?
A)
Tomato and wheat
done
clear
B)
Tomato and rice
done
clear
C)
Tobacco and tomato
done
clear
D)
Maize and sugarcane
done
clear
View Answer play_arrow
question_answer 199) Foetal abnormalities are caused by
A)
LSD
done
clear
B)
opium
done
clear
C)
nicotine
done
clear
D)
alcohol
done
clear
View Answer play_arrow
question_answer 200) Hybridoma is a bio-technique which involves fusion of
A)
B-cells and T-cells
done
clear
B)
T-cells and spleen cells
done
clear
C)
spleen cells and myeloma
done
clear
D)
myeloma cell and B-cells
done
clear
View Answer play_arrow