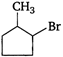
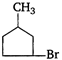
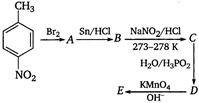
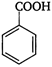
question_answer 1) Which of the following does not depict the correct link between technology and physics?
A)
Optical fibres \[\leftrightarrow \] total internal reflection of light
done
clear
B)
Nuclear reactor \[\leftrightarrow \] nuclear fusion
done
clear
C)
Electron microscope \[\leftrightarrow \] wave nature of electrons
done
clear
D)
Electric generator \[\leftrightarrow \] laws of electromagnetic induction
done
clear
View Answer play_arrow
question_answer 2) If E, M, L and G denote energy, mass, angular momentum and gravitational constant respectively, then the quantity \[({{\text{E}}^{\text{2}}}{{\text{L}}^{\text{2}}}/{{\text{M}}^{\text{5}}}{{\text{G}}^{\text{2}}})\]has the dimensions of
A)
angle
done
clear
B)
length
done
clear
C)
mass
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 3) Choose the incorrect statement out of the following.
A)
Every measurement by any measuring instrument has some error
done
clear
B)
Every calculated physical quantity that is based on measured values has some error
done
clear
C)
A measurement can have more accuracy but less precision and vice-versa
done
clear
D)
The percentage error is different from relative error
done
clear
View Answer play_arrow
question_answer 4) Select the incorrect statements from the following S1 : Average velocity is path length divided by time interval. S2 : In general, speed is greater than the magnitude of the velocity. S3 : A particle moving in a given direction with a non-zero velocity can have zero speed. S4 : The magnitude of average velocity is the average speed.
A)
\[{{S}_{2}}\]and \[{{S}_{3}}\]
done
clear
B)
\[{{S}_{1}}\]and \[{{S}_{4}}\]
done
clear
C)
\[{{S}_{1}},\text{ }{{S}_{3}}\]and \[{{S}_{4}}\]
done
clear
D)
All four statements
done
clear
View Answer play_arrow
question_answer 5) The ratios of the distance traversed, in successive intervals of time by a body, falling from rest, are
A)
1 : 3 : 5 : 7 : 9.........
done
clear
B)
2 : 4 : 6 : 8 : 10 .........
done
clear
C)
1 : 4 : 7 : 10 : 13 .........
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 6) A body A is thrown up vertically from the ground with a velocity \[{{v}_{o}}\]and another body B is simultaneously dropped from a height H. They meet at a height \[\frac{H}{2}\] if \[{{v}_{o}}\] is equal to
A)
\[\sqrt{2gH}\]
done
clear
B)
\[\sqrt{gH}\]
done
clear
C)
\[\frac{1}{2}\sqrt{gH}\]
done
clear
D)
\[\frac{2g}{H}\]
done
clear
View Answer play_arrow
question_answer 7)
Which of the following graphs cannot possibly represent one dimensional motion of a particle?
A)
I and II
done
clear
B)
II and III
done
clear
C)
II and IV
done
clear
D)
All four
done
clear
View Answer play_arrow
question_answer 8) A block of mass m is placed on a smooth sphere of radius R. It slides when pushed slightly. At what distance h, from the top, will it leave the sphere?
A)
\[\frac{R}{4}\]
done
clear
B)
\[\frac{R}{3}\]
done
clear
C)
\[\frac{R}{2}\]
done
clear
D)
\[R\]
done
clear
View Answer play_arrow
question_answer 9) A man, of mass 60 kg, is riding in a lift. The weight of the man, when the lift is accelerating upwards and downwards at 2 \[m{{s}^{-2}}\] are respectively \[\left( \text{taking g }=\text{ 1}0\text{ m}{{\text{s}}^{-\text{2}}} \right)\]
A)
720 N and 480 N
done
clear
B)
480 N and 720 N
done
clear
C)
600 N and 600 N
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 10) One end of a string of length L is connected to a particle of mass M and the other to a small peg on a smooth horizontal table. If the particle moves in a circle with a speed v, the net force, directed towards the centre, on the particle is
A)
\[T+\frac{M{{v}^{2}}}{L}\]
done
clear
B)
\[T\]
done
clear
C)
\[T-\frac{M{{v}^{2}}}{L}\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 11) A variable force, given by the two- dimensional vector \[\text{F }=\left( \text{3}{{\text{x}}^{\text{2}}}\overset{\hat{\ }}{\mathop{\text{i}}}\,+\text{4}\overset{\hat{\ }}{\mathop{\text{j}}}\, \right),\] acts on a particle. The force is in newton and x is in metre. What is the change in the kinetic energy of the particle as it moves from the point with coordinates (2, 3) to (3, 0)? (The coordinates are in metre)
A)
-7 J
done
clear
B)
Zero
done
clear
C)
+7 J
done
clear
D)
d) +19 J
done
clear
View Answer play_arrow
question_answer 12) A billiards player hits a stationary ball by an identical ball to pocket the target ball in a comer pocket that is at an angle of \[35{}^\circ \] with respect to the direction of motion of the first ball. Assuming the collision as elastic and that frictional and rotational motions are not important, the angle made by the target ball with respect to the incoming ball is
A)
\[35{}^\circ \]
done
clear
B)
\[50{}^\circ \]
done
clear
C)
\[55{}^\circ \]
done
clear
D)
\[60{}^\circ \]
done
clear
View Answer play_arrow
question_answer 13) The centre of mass of a system of three particles of masses 1 g, 2 g and 3 g is taken as the origin of a coordinate system. The position vector of a fourth particle of mass 4 g such that the centre of mass of the four particle system lies at the point (1, 2, 3) is a \[\left( \overset{\hat{\ }}{\mathop{\text{i}}}\,+\text{2 }\overset{\hat{\ }}{\mathop{\text{j}}}\,+\text{3}\,\overset{\hat{\ }}{\mathop{\text{k}}}\, \right),\] where a is a constant. The value of a is
A)
\[\frac{10}{3}\]
done
clear
B)
\[\frac{5}{2}\]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
\[\frac{2}{5}\]
done
clear
View Answer play_arrow
question_answer 14) A string is wound round the rim of a mounted flywheel of mass 20 kg and radius 20 cm. A steady pull of 25 N is applied on the cord. Neglecting friction and mass of the string, the angular acceleration of the wheel is
A)
\[\text{5}0{{\text{s}}^{-\text{2}}}\]
done
clear
B)
\[\text{25}{{\text{s}}^{-\text{2}}}\]
done
clear
C)
\[\text{12}.\text{5}{{\text{s}}^{-\text{2}}}\]
done
clear
D)
\[\text{6}.\text{25}{{\text{s}}^{-\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 15) A person, with outstretched arms, is spinning on a rotating stool. He suddenly brings his arms down to his sides. Which the following is true about his kinetic energy K and angular momentum L?
A)
Both K and L increase
done
clear
B)
Both K and L remain unchanged
done
clear
C)
K remains constant, L increases
done
clear
D)
K increases but L remains constant
done
clear
View Answer play_arrow
question_answer 16) Three equal masses of 1 kg each are placed at the vertices of an equilateral triangle PQ.R and a mass of 2 kg is placed at the centroid 0 of the triangle which is at a distance of V2 m from each of the vertices of the triangle. The force, in newton, acting on the mass of 2 kg is
A)
2
done
clear
B)
\[\sqrt{2}\]
done
clear
C)
1
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 17) One can easily weighs the earth by calculating the mass of earth using the formula (in usual notation)
A)
\[\frac{G}{g}R_{E}^{2}\]
done
clear
B)
\[\frac{g}{G}R_{E}^{2}\]
done
clear
C)
\[\frac{g}{G}R_{{}}^{2}\]
done
clear
D)
\[\frac{G}{g}R_{E}^{3}\]
done
clear
View Answer play_arrow
question_answer 18) Choose the correct statement(s) for a cricket ball that is spinning clockwise through air S1 : Streamlines of air are symmetric around the ball. S2: The velocity of air above the ball relative to it is larger than that below the ball. 53 : The velocity of air above the ball relative to it is smaller than that below the ball. S4 : There is a net upward force on the ball.
A)
S1, S2 and S4
done
clear
B)
S2 and S4
done
clear
C)
S4 only
done
clear
D)
S3 only
done
clear
View Answer play_arrow
question_answer 19) Adding detergents to water helps in removing dirty greasy stains. This is because it increases the oil-water surface tension it decreases the oil-water surface tension it increases the viscosity of the solution dirt is held suspended surrounded by detergent molecules
A)
2 and 4
done
clear
B)
1 only
done
clear
C)
3 and 4
done
clear
D)
4 only
done
clear
View Answer play_arrow
question_answer 20) During an adiabatic expansion, the increase in volume is associated with which of the following possibilities w.r.t. pressure and temperature?
A)
Pressure Temperature increase increase
done
clear
B)
decrease decrease
done
clear
C)
increase decrease
done
clear
D)
decrease increase
done
clear
View Answer play_arrow
question_answer 21) Choose the incorrect statement from the following S1 : The efficiency of a heat engine can be 1, but the coefficient of performance of a refrigerator can never be infinity. S2: The first law of thermodynamics is basically the principle of conservation of energy. S3: The second law of thermodynamics does not allow several phenomena Consistent with the first law S4: A process, whose sole result is the transfer of heat from a colder object to a hotter object is impossible.
A)
S1
done
clear
B)
S3
done
clear
C)
S2
done
clear
D)
S4
done
clear
View Answer play_arrow
question_answer 22) A cylinder of fixed capacity (of 44.8 L) contains 2 moles of helium gas at STP. What is the amount of heat needed to raise the temperature of the gas in the cylinder by \[{{20}^{o}}C?(Use\,R=8.31\,Jmo{{l}^{-1}}{{K}^{-1}})\]
A)
996 J
done
clear
B)
831 J
done
clear
C)
498 J
done
clear
D)
374 J
done
clear
View Answer play_arrow
question_answer 23) What is the type of motion of a mass if the total force acting on the mass at a time \[t\,is\,\overset{\to }{\mathop{F}}\,=-k\,\overset{\to }{\mathop{x}}\,-b\,\overset{\to }{\mathop{v}}\,\,,\]where k and b are constants?
A)
Simple harmonic motion
done
clear
B)
Forced oscillations
done
clear
C)
Resonance
done
clear
D)
Damped harmonic oscillations
done
clear
View Answer play_arrow
question_answer 24) A travelling wave is represented by the equation \[\text{y}=\frac{1}{10}\text{ sin}\left( \text{6}0\text{t}+\text{2x} \right),\]where x and \[\text{y}\]are in metre and c is in second. This represents a wave 1. travelling with a velocity of 30 \[\text{m}{{\text{s}}^{\text{-1}}}\]30 2. of frequency \[\frac{30}{\pi }\] Hz 3. of wavelength 7t metre 4. of amplitude 10 cm 5. moving in the positive x - direction Pick out the correct statements from the above.
A)
1, 2, 4
done
clear
B)
3, 4, 5
done
clear
C)
1,2,3,4
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 25) Gausss law of electrostatics would be invalid if
A)
there were magnetic monopoles
done
clear
B)
the speed of light was not a universal constant
done
clear
C)
the inverse square law was not exactly true
done
clear
D)
the electrical charge was not quantized
done
clear
View Answer play_arrow
question_answer 26) Consider the following statements about electric dipole and select the correct ones. S1 : Electric dipole moment vector \[\overset{\to }{\mathop{p}}\,\]is directed from the negative charge to the positive charge 52 : The electric field of a dipole at a point with position vector \[\overset{\to }{\mathop{r}}\,\] depends on \[\overset{\to }{\mathop{\left| \text{r} \right|}}\,\] as well as the angle between r and p. The electric dipole potential falls off as \[\frac{1}{{{r}^{2}}}\] and not as \[\frac{1}{r}.\] S4: In a uniform electric field, the electric dipole experiences no net forces but a torque \[\overset{\to }{\mathop{\text{t}}}\,=\overset{\to }{\mathop{\text{p}}}\,\text{ x }\overset{\to }{\mathop{\text{E}}}\,.\]
A)
S2, S3 and S4
done
clear
B)
S3 and S4
done
clear
C)
S2 and S3
done
clear
D)
All four
done
clear
View Answer play_arrow
question_answer 27) A slab of material of dielectric constant K has the same area as the plates of a parallel plate capacitor but has a thickness \[\left( \frac{3}{4} \right)\] d, where d is the separation of the plates. The ratio of the capacitance C (in the presence of the dielectric) to the capacitance \[{{C}_{o}}\](in the absence of the dielectric) is
A)
\[\frac{3K}{K+3}\]
done
clear
B)
\[\frac{3}{4}K\]
done
clear
C)
\[\frac{4K}{K+3}K\]
done
clear
D)
\[\frac{4}{3}K\]
done
clear
View Answer play_arrow
question_answer 28) A color coded carbon resistor has the colors orange, blue, green and silver. Its resistance value and tolerance percentage respectively are
A)
\[\text{36}\times \text{l}{{\text{0}}^{5}}\Omega \text{ and 1}0%\]
done
clear
B)
\[\text{36}\times \text{l}{{\text{0}}^{4}}\Omega \text{ and 5}%\]
done
clear
C)
\[63\times {{10}^{5}}\Omega \,and\,10%\]
done
clear
D)
\[\text{35}\times \text{l}{{0}^{\text{6}}}\,\Omega \,\text{and}\,\text{5}%\]
done
clear
View Answer play_arrow
question_answer 29)
For the current loops shown in the figure, Kirchhoffs loop rule for the loops AHDCBA and AHDEFGA yields these equations respectively
A)
\[-30{{I}_{1}}-41{{I}_{3}}+45=0\,\,and\,-30A{{I}_{1}}\] \[+21{{I}_{2}}-80=0\]
done
clear
B)
\[30{{I}_{1}}-41{{I}_{3}}+45=0\,\,and\]\[30{{I}_{1}}-21{{I}_{2}}-80=0\]
done
clear
C)
\[30{{I}_{1}}+41{{I}_{3}}-45=0\,\,and\,\]\[-30{{I}_{1}}+21{{I}_{3}}+80=0\,\,\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 30) In an experiment with potentiometer, a cell of emf 1.25 V gives a balance point at 35 cm length of the wire. If the cell is replaced by another cell, the balance point shift to 63 cm. The emf of the second cell is
A)
3.25 V
done
clear
B)
2.5 V
done
clear
C)
2.25 V
done
clear
D)
2 V
done
clear
View Answer play_arrow
question_answer 31) The magnitude of the magnetic field required to accelerate protons (mass \[=\text{1}.\text{67x 1}{{0}^{-\text{27}}}\text{ kg})\]in a cyclotron that is operated at an oscillator frequency 12 MH is approximately
A)
0.8 T
done
clear
B)
1.6 T
done
clear
C)
2.0 T
done
clear
D)
3.2 T
done
clear
View Answer play_arrow
question_answer 32)
Each of the three parallel wires, A, B and C are carrying current in the direction shown in the figure. The wire B will
A)
move to the right
done
clear
B)
move to the left
done
clear
C)
remain stationary
done
clear
D)
moved down in the direction of the current
done
clear
View Answer play_arrow
question_answer 33) A magnet is placed in a non-uniform magnetic field. It experiences
A)
a force but not a torque
done
clear
B)
a torque but not a force
done
clear
C)
a force and a torque
done
clear
D)
neither a force nor a torque
done
clear
View Answer play_arrow
question_answer 34) The north pole of a long bar magnet was pushed slowly into a short solenoid connected to a galvanometer. The magnet was held stationary for a few seconds with the North Pole in the middle of the solenoid and then withdrawn rapidly. The maximum deflection of the galvanometer was observed when the magnet was
A)
moving towards the solenoid
done
clear
B)
moving into the solenoid
done
clear
C)
at rest inside the solenoid
done
clear
D)
moving out of the solenoid
done
clear
View Answer play_arrow
question_answer 35) A light bulb is rated 100 W for a 220 V supply. The resistance of the bulb and the peak voltage of the source respectively are
A)
2420 and 311 V
done
clear
B)
4840 and 311 V
done
clear
C)
484 Q and 440 V
done
clear
D)
242 Q and 440 V
done
clear
View Answer play_arrow
question_answer 36) A pure inductor of 25 mH is connected to a source of 220 V. Given the frequency of the source as 50 Hz, the rms current in the circuit is
A)
7 A
done
clear
B)
14 A
done
clear
C)
28 A
done
clear
D)
42 A
done
clear
View Answer play_arrow
question_answer 37) Consider the following statements about electromagnetic waves and choose the correct ones. S1 : EM waves having wavelengths 1000 times smaller than light waves are called X-rays. S2 : Ultraviolet waves are used in the treatment of swollen joints. S3 : Alpha and gamma rays are not electromagnetic waves. S4 : de-Broglie waves are not electromagnetic in nature. S5: Electromagnetic waves exhibit polarization while sound waves do not.
A)
S1, S4 and S5
done
clear
B)
S3, S4 and S5
done
clear
C)
S1, S3 and S5
done
clear
D)
S2, S3 and S4
done
clear
View Answer play_arrow
question_answer 38) Ray optics fails when
A)
the size of the obstacle is 5 cm
done
clear
B)
the size of the obstacle is 3 cm
done
clear
C)
the size of the obstacle is less than the wavelength of light
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 39) Pick out rhe correct statements about optical fibres from the following. S1 : Optical fibres are used for the transmission of optical signals only. S2 : Optical fibres are used for transmitting and receiving electrical signals. S3 : The intensity of light signals sent through optical fibres suffer very small loss. S4: Optical fibres effectively employ the principle of multiple total internal reflections. S5 : Optical fibres are glass fibres coated with a thin layer of a material with lower refractive index.
A)
S1 and S2
done
clear
B)
S2 and S3
done
clear
C)
S3 and S4
done
clear
D)
S2, S3, S4 and S5
done
clear
View Answer play_arrow
question_answer 40) The rectilinear propagation of light in a medium is due to its
A)
high velocity
done
clear
B)
large wavelength
done
clear
C)
high frequency
done
clear
D)
source
done
clear
View Answer play_arrow
question_answer 41) Check the correct statements on scattering of light. S1 : Rayleigh scattering is responsible for the bluish appearance of sky. S2 : Rayleigh scattering is proportional to \[1/{{\lambda }^{4}}\]when the size of the scatterer is much less than\[\lambda \]. S3 : Clouds having droplets of water (large scattering objects) scatter all wavelengths are almost equal and so are generally white. S4 : The sun looks reddish at sunset and sunrise due to Rayleigh scattering.
A)
S1 only
done
clear
B)
S1 and S2
done
clear
C)
S2 and S3
done
clear
D)
S1, S2, S3 and S4
done
clear
View Answer play_arrow
question_answer 42) A diffraction pattern is obtained using a beam of red light. What happens if the red light is replaced by blue light?
A)
No change
done
clear
B)
Diffraction bands become narrower and crowded together
done
clear
C)
Bands become broader and farther apart
done
clear
D)
Bands disappear altogether
done
clear
View Answer play_arrow
question_answer 43) Light of two different frequencies, whose photons have energies 1 eV and 2.5 eV are respectively, successively illuminate a surface of work function 0.5 eV. The ratio of the maximum speeds of the electrons that are emitted is
A)
2 : 1
done
clear
B)
1 : 2
done
clear
C)
1 : 3
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 44) The ratio of the de-Broglie wavelength of an electron of energy 10 eV to that of person of mass 66 kg travelling at a speed of 100 km/h is of the order of
A)
\[\text{1}{{0}^{\text{34}}}\]
done
clear
B)
\[\text{1}{{0}^{\text{27}}}\]
done
clear
C)
\[\text{1}{{0}^{\text{17}}}\]
done
clear
D)
\[\text{1}{{0}^{-\text{1}0}}\]
done
clear
View Answer play_arrow
question_answer 45) According to the Bohr theory of hydrogen atom, the speed of the electron, its energy and the radius of its orbit varies with the principal quantum number n, respectively, as
A)
\[\frac{1}{n},\frac{1}{{{n}^{2}}},{{n}^{2}}\]
done
clear
B)
\[\frac{1}{n},{{n}^{2}},\frac{1}{{{n}^{2}}}\]
done
clear
C)
\[{{n}^{2}},\frac{1}{{{n}^{2}}},{{n}^{2}}\]
done
clear
D)
\[n,\frac{1}{{{n}^{2}}},\frac{1}{{{n}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 46) Consider the following statements. S1 : The nuclear force is independent of the charge of nucleons. S2 : The number of nucleons in the nucleus of an atom is equal to the number of electrons in the atom. S3 : All nuclei have masses that are less than the sum of the masses of constituent nucleons. S4 : Nucleons belong to the family of leptons while electrons are members of the family of hadrons. Choose the correct statement (s) from these
A)
S1 only
done
clear
B)
S1 and S4
done
clear
C)
S2, S3 and S4
done
clear
D)
S1 and S3
done
clear
View Answer play_arrow
question_answer 47) The crucial function of moderators in nuclear reactors is to
A)
decrease the energy of neutrons
done
clear
B)
absorb the extra neutrons
done
clear
C)
provide shield from nuclear radiations
done
clear
D)
provide cooling
done
clear
View Answer play_arrow
question_answer 48) An n-p-n transistor is biased to work as an amplifier. Which of the following statements is false?
A)
The electrons go from base region to the collector region
done
clear
B)
The electrons go from the collector region to the base region
done
clear
C)
The electrons go from emitter region to the base region
done
clear
D)
The electrons go from base region to the emitter region
done
clear
View Answer play_arrow
question_answer 49) In a common base amplifier, the phase difference between the input signal voltage and the output voltage is
A)
0
done
clear
B)
\[\pi /4\]
done
clear
C)
\[\pi /2\]
done
clear
D)
\[\pi \]
done
clear
View Answer play_arrow
question_answer 50) Arrange the following communication frequency bands in the increasing order of frequencies AM broadcast Cellular mobile radio FM broadcast Television UHF Satellite communication
A)
1 3 4 2 5
done
clear
B)
1 2 3 4 5
done
clear
C)
5 2 4 3 1
done
clear
D)
1 3 2 4 5
done
clear
View Answer play_arrow
question_answer 51) When the electron of a hydrogen atom jumps from n = 4 to n = 1 state, the number of spectral lines emitted is
A)
15
done
clear
B)
9
done
clear
C)
6
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 52)
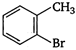
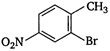
Which of the following is an appropriate set of reactants for the preparation of 1 -methoxy-4 nitrobenzene?
A)
A
done
clear
B)
B
done
clear
C)
Both A and B
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 53) Consider the decomposition of \[{{N}_{2}}{{O}_{5}}\] as \[{{N}_{2}}{{O}_{5}}\xrightarrow{{}}2N{{O}_{2}}+\frac{1}{2}{{O}_{2}}\] The rate of reaction is given by \[\frac{-d[{{N}_{2}}{{O}_{5}}]}{dt}=\frac{1}{2}\frac{d[N{{O}_{2}}]}{dt}=2\frac{d[{{O}_{2}}]}{dt}={{k}_{1}}[{{N}_{2}}{{O}_{5}}]\] Therefore, \[\frac{-d[{{N}_{2}}{{O}_{5}}]}{dt}={{k}_{1}}[{{N}_{2}}{{O}_{5}}]\] \[\frac{+d\,[N{{O}_{2}}]}{dt}=2{{k}_{1}}[{{N}_{2}}{{O}_{5}}]=k_{1}^{}[{{N}_{2}}{{O}_{5}}]\] \[\frac{+d\,\,[{{O}_{2}}]}{dt}=\frac{1}{2}{{k}_{1}}[{{N}_{2}}{{O}_{5}}]=k_{1}^{}[{{N}_{2}}{{O}_{5}}]\] Choose the correct option.
A)
\[{{k}_{1}}=k{{}_{1}}={{k}_{1}}\]
done
clear
B)
\[{{k}_{1}}=2k_{1}^{}=k_{1}^{}\]
done
clear
C)
\[4{{k}_{1}}=k_{1}^{}=2k_{1}^{}\]
done
clear
D)
\[4{{k}_{1}}=2k_{1}^{}=k_{1}^{}\]
done
clear
View Answer play_arrow
question_answer 54) The logarithm of the equilibrium constant of the cell reaction corresponding to the cell\[X(s)|{{X}^{2+}}(aq)||{{Y}^{+}}(aq)|\,Y(s)\] with standard cell potential, \[E_{cell}^{o}=1.2\,V\] is given by
A)
12.5
done
clear
B)
21.5
done
clear
C)
40.5
done
clear
D)
47.2
done
clear
View Answer play_arrow
question_answer 55) If the half cell reactions are given as (i) \[F{{e}^{2+}}(aq)+2{{e}^{-}}\to Fe(s);\,\,\,\,{{E}^{o}}=-0.44\,\,V\] (ii) \[2{{H}^{+}}(aq)+\frac{1}{2}{{O}_{2}}(g)+2{{e}^{-}}\to {{H}_{2}}O(l)\] \[{{E}^{o}}=+1.23\,\,V\] The \[{{E}^{o}}\] for the reaction \[Fe(s)+2{{H}^{+}}+\frac{1}{2}{{O}_{2}}(g)\to F{{e}^{2+}}(aq)+{{H}_{2}}O(l)\] is
A)
+ 1.67V
done
clear
B)
-1.67V
done
clear
C)
+ 0.79V
done
clear
D)
-0.79V
done
clear
View Answer play_arrow
question_answer 56) The most adsorbed gas on activated charcoal is
A)
\[{{N}_{2}}\]
done
clear
B)
\[{{H}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 57) Which of the following represents the arrangement in increasing order of bond order and bond dissociation energy?
A)
\[O_{2}^{+}<O_{2}^{2-}<O_{2}^{-}<{{O}_{2}}\]
done
clear
B)
\[O_{2}^{2-}<O_{2}^{-}<{{O}_{2}}<O_{2}^{+}\]
done
clear
C)
\[{{O}_{2}}<O_{2}^{+}<O_{2}^{2-}<O_{2}^{-}\]
done
clear
D)
\[O_{2}^{2-}<O_{2}^{-}<O_{2}^{+}<{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 58) AgCl is dissolved in excess of each of \[N{{H}_{3}}\], KCN and \[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]. The complex ions produced in each case are
A)
\[{{[Ag{{(N{{H}_{3}})}_{2}}]}^{+}},{{[Ag{{(CN)}_{2}}]}^{+}}\]and \[{{[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}]}^{3-}}\]
done
clear
B)
\[{{[Ag{{(N{{H}_{3}})}_{2}}]}^{2+}},{{[Ag{{(CN)}_{2}}]}^{3-}}\] and \[{{[A{{g}_{2}}{{({{S}_{2}}{{O}_{3}})}_{2}}]}^{3-}}\]
done
clear
C)
\[Ag{{(N{{H}_{3}})}_{4}}{{]}^{2+}},{{[Ag{{(CN)}_{2}}]}^{3-}}\] and \[{{[A{{g}_{2}}{{({{S}_{2}}{{O}_{3}})}_{2}}]}^{3-}}\]
done
clear
D)
\[{{[Ag{{(N{{H}_{3}})}_{2}}]}^{+}},{{[Ag{{(CN)}_{2}}]}^{-}}\] and \[{{[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}]}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 59) The most stable complex among the following is
A)
\[{{[Pd{{(CN)}_{4}}]}^{4-}}\]
done
clear
B)
\[[Fe{{(CO)}_{5}}]\]
done
clear
C)
\[{{[Ni{{(CN)}_{4}}]}^{4-}}\]
done
clear
D)
\[{{[Ni{{(CN)}_{4}}]}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 60) The maximum number of P?H bonds are contained in which of the following molecules?
A)
\[{{H}_{3}}P{{O}_{2}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
D)
\[{{H}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
View Answer play_arrow
question_answer 61) The bond order of the N?0 bonds in \[NO_{3}^{-}\] ion is
A)
0.33
done
clear
B)
1.00
done
clear
C)
1.33
done
clear
D)
1.50
done
clear
View Answer play_arrow
question_answer 62) Among the following the third ionization energy is highest for
A)
magnesium
done
clear
B)
boron
done
clear
C)
beryllium
done
clear
D)
aluminium
done
clear
View Answer play_arrow
question_answer 63) The highest lattice energy corresponds to
A)
MgO
done
clear
B)
CaO
done
clear
C)
SrO
done
clear
D)
BaO
done
clear
View Answer play_arrow
question_answer 64) The change of energy on freezing 1.00 kg of liquid water at \[{{0}^{o}}C\] and 1 atm is
A)
236.7 \[kJ\,k{{g}^{-1}}\]
done
clear
B)
333.4 \[kJ\,k{{g}^{-1}}\]
done
clear
C)
-333.4 \[kJ\,k{{g}^{-1}}\]
done
clear
D)
-236.7 \[kJ\,k{{g}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 65) The degree of hardness of water is usually expressed in terms of
A)
ppm by weight of \[MgS{{O}_{4}}\]
done
clear
B)
g/L of \[CaC{{O}_{3}}\]and \[MgS{{O}_{3}}\] present
done
clear
C)
ppm by weight of \[CaC{{O}_{3}}\] irrespective of whether it is actually present
done
clear
D)
ppm of \[CaC{{O}_{3}}\] actually present in water
done
clear
View Answer play_arrow
question_answer 66) 0.1 M NaCI and 0.05 M \[BaC{{l}_{2}}\] solutions are separated by a semipermeable membrane in a container. For this system, choose the correct answer.
A)
There is no movement of any solution across the membrane
done
clear
B)
Water flows from \[BaC{{l}_{2}}\] solution towards NaCI solution
done
clear
C)
Water flows from NaCI solution towards \[BaC{{l}_{2}}\] solution
done
clear
D)
Osmotic pressure of 0.1 M NaCI is lower than the osmotic pressure of \[BaC{{l}_{2}}\] (Assume complete dissociation)
done
clear
View Answer play_arrow
question_answer 67) If W is the amount of work done by the system and q is the amount of heat supplied to the system, identify the type of the system.
A)
Isolated system
done
clear
B)
Closed system
done
clear
C)
Open system
done
clear
D)
System with thermally conducting walls
done
clear
View Answer play_arrow
question_answer 68) The charge balance equation of species in 0.100 M acetic acid solution is given by
A)
\[[{{H}^{+}}]=[O{{H}^{-}}]\]
done
clear
B)
\[[{{H}^{+}}]=[C{{H}_{3}}CO{{O}^{-}}]\]
done
clear
C)
\[[{{H}^{+}}]=[O{{H}^{-}}]+[C{{H}_{3}}CO{{O}^{-}}]\]
done
clear
D)
\[2[{{H}^{+}}]=[O{{H}^{-}}]+[C{{H}_{3}}CO{{O}^{-}}]\]
done
clear
View Answer play_arrow
question_answer 69) Total number or voids in 0.5 mole or compound forming hexagonal closed packed structure are
A)
\[6.022\times {{10}^{23}}\]
done
clear
B)
\[3.011\times {{10}^{23}}\]
done
clear
C)
\[9.033\times {{10}^{23}}\]
done
clear
D)
\[4.516\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 70) Four solutions of \[{{K}_{2}}S{{O}_{4}}\] with the following concentration 0.1 M, 0.01 m, 0.001 m and m are available. The maximum value of vant Hoff factor, i, corresponds to
A)
0.0001 m solution
done
clear
B)
0.001 m solution
done
clear
C)
0.01 m solution
done
clear
D)
0.1 m solution
done
clear
View Answer play_arrow
question_answer 71) The pH of a solution prepared by mixing 2.0 mL of HCl solution of pH 3.0 and 3.0 mL of NaOH of pH 10.0 is
A)
2.5
done
clear
B)
3.5
done
clear
C)
5.5
done
clear
D)
6.5
done
clear
View Answer play_arrow
question_answer 72) The set of quantum numbers that represents the highest energy of an atom is
A)
\[n=4,\,\,l=0,\,\,m=0,\,\,s=+\frac{1}{2}\]
done
clear
B)
\[n=3,\,\,l=2,\,\,m=1,\,\,s=+\frac{1}{2}\]
done
clear
C)
\[n=3,\,\,l=1,\,\,m=1,\,\,s=+\frac{1}{2}\]
done
clear
D)
\[n=3,\,\,l=0,\,\,m=0,\,\,s=+\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 73) Which of the following sets of quantum numbers represents the 19th electron in chromium? (Z = 24 for Cr)
A)
4, 0, 0 \[\frac{1}{2}\]
done
clear
B)
4, 1, -1, \[\frac{1}{2}\]
done
clear
C)
3, 2, 2, \[\frac{1}{2}\]
done
clear
D)
3, 2, -2, \[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 74) The number of molecules in 100 mL of N \[{{H}_{2}}S{{O}_{4}}\] is
A)
\[6.022\times {{10}^{22}}\]
done
clear
B)
\[6.02\times {{10}^{21}}\]
done
clear
C)
\[6.02\times {{10}^{20}}\]
done
clear
D)
\[6.02\times {{10}^{18}}\]
done
clear
View Answer play_arrow
question_answer 75) For the reaction \[AB(g)A(g)+B(g),\,AB\] is 33% dissociated at a total pressure of p. Therefore, p is related to \[{{K}_{p}}\] by one of the following options
A)
\[p={{K}_{p}}\]
done
clear
B)
\[p=3{{K}_{p}}\]
done
clear
C)
\[p=4{{K}_{p}}\]
done
clear
D)
\[p=8{{K}_{p}}\]
done
clear
View Answer play_arrow
question_answer 76) When subjected to acid catalysed hydration, the order of reactivity of the alkenes; \[{{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}(I),C{{H}_{3}}CH=C{{H}_{2}}(II)\] and\[C{{H}_{2}}=C{{H}_{2}}(III)\] is
A)
III > II > I
done
clear
B)
I > III > II
done
clear
C)
I > II > III
done
clear
D)
II > I > III
done
clear
View Answer play_arrow
question_answer 77) Name the reagent used to bring about the following transformation : But-2-ene to ethanal
A)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] in acidic medium
done
clear
B)
\[Cr{{O}_{2}}C{{l}_{2}}/{{H}_{3}}{{O}^{+}}\]
done
clear
C)
PCC
done
clear
D)
\[{{C}_{3}}/{{H}_{2}}O\] -Zn dust
done
clear
View Answer play_arrow
question_answer 78) Arrange the following in increasing order of their basic strength : \[C{{H}_{3}}N{{H}_{2}}(I),\,{{(C{{H}_{3}})}_{2}}NH(II),{{(C{{H}_{3}})}_{3}}N(III)\],\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}N{{H}_{2}}(IV)\]
A)
IV < III < II < I
done
clear
B)
IV < III < I < II
done
clear
C)
I < II < III < IV
done
clear
D)
IV < III < I = II
done
clear
View Answer play_arrow
question_answer 79) Arrange the following in increasing order of their intermolecular forces : Nylon-66 (I), Buna-S (II), Polythene (III)
A)
II, I, III
done
clear
B)
III, II, I
done
clear
C)
I, II, III
done
clear
D)
II, III, I
done
clear
View Answer play_arrow
question_answer 80) The \[p{{K}_{{{a}_{1}}}}\] and \[p{{K}_{{{a}_{2}}}}\]of an amino acid are 2.3 and 9.7 respectively. The isoelectric point of the amino acid is
A)
12.0
done
clear
B)
7.4
done
clear
C)
6.0
done
clear
D)
3.7
done
clear
View Answer play_arrow
question_answer 81) The transfer RNA anticodon for the messenger RNA codon G-C-A is
A)
C-G-U
done
clear
B)
G-C-U
done
clear
C)
U-G-C
done
clear
D)
G-U-C
done
clear
View Answer play_arrow
question_answer 82) The number of p-particles emitted during the transformation of \[_{y}^{x}A\] to \[_{n}^{m}B\]
A)
\[\frac{x-m}{4}\]
done
clear
B)
\[n+\frac{x-m}{2}+y\]
done
clear
C)
\[n+\frac{x-m}{2}-y\]
done
clear
D)
\[2y-n+x-m\]
done
clear
View Answer play_arrow
question_answer 83)
In the following reaction,
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 84)
Identify the product (E) in the following sequence of reactions.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 85) Which of the following fluoride of xenon has zero dipole moment?
A)
\[Xe{{F}_{2}}\]
done
clear
B)
\[Xe{{F}_{3}}\]
done
clear
C)
\[Xe{{F}_{4}}\]
done
clear
D)
\[Xe{{F}_{6}}\]
done
clear
View Answer play_arrow
question_answer 86) Choose the correct statement.
A)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{2+}}\] is oxidised to diamagnetic \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] by the oxygen in air
done
clear
B)
Tetrahedral complexes are more stable than octahedral complexes
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{3-}}\] is stable but \[{{[Fe{{F}_{6}}]}^{3-}}\] is unstable
done
clear
D)
The \[{{[Cu{{[N{{H}_{3}})}_{4}}]}^{2+}}\] ion has a tetrahedral geometry and is diamagnetic
done
clear
View Answer play_arrow
question_answer 87) The hydrolysis of \[NC{{l}_{3}}\] by water produces
A)
\[N{{H}_{2}}OH\] and HOCI
done
clear
B)
\[N{{H}_{2}}N{{H}_{2}}\] and HCl
done
clear
C)
\[N{{H}_{4}}OH\] and HOCl
done
clear
D)
\[N{{H}_{2}}Cl\] and HOCI
done
clear
View Answer play_arrow
question_answer 88) During the conversion of \[N{{H}_{2}}OH\to {{N}_{2}}O\], the equivalent weight of \[N{{H}_{2}}OH\] (mol. wt. of \[N{{H}_{2}}OH\] is M) is
A)
M
done
clear
B)
M/2
done
clear
C)
M/4
done
clear
D)
M/5
done
clear
View Answer play_arrow
question_answer 89) In the disintegration process\[A\xrightarrow{-\alpha }B\xrightarrow{-\beta }C\xrightarrow{-\beta }D\]the correct statement is
A)
A and B are isobars
done
clear
B)
A and C are isotones
done
clear
C)
A and B are isotopes
done
clear
D)
A and D are isotopes
done
clear
View Answer play_arrow
question_answer 90) Which of the following compounds shows both Frenkel and Schottky defects?
A)
NaCI
done
clear
B)
AgCl
done
clear
C)
AgBr
done
clear
D)
KCl
done
clear
View Answer play_arrow
question_answer 91) The pH of a solution obtained by mixing equal volumes of \[\frac{N}{10}\] NaOH and \[\frac{N}{20}\] HCl is
A)
13.4
done
clear
B)
12.4
done
clear
C)
7.6
done
clear
D)
1.6
done
clear
View Answer play_arrow
question_answer 92) The solubilities of \[N{{a}_{2}}S{{O}_{4}},BeS{{O}_{4}},\,MgS{{O}_{4}}\] and \[BeS{{O}_{4}}\] will follow the order
A)
\[BeS{{O}_{4}}>MgS{{O}_{4}}>N{{a}_{2}}S{{O}_{4}}>BaS{{O}_{4}}\]
done
clear
B)
\[BeS{{O}_{4}}>N{{a}_{2}}S{{O}_{4}}>MgS{{O}_{4}}>BaS{{O}_{4}}\]
done
clear
C)
\[MgS{{O}_{4}}>BeS{{O}_{4}}>N{{a}_{2}}S{{O}_{4}}>BaS{{O}_{4}}\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}>BeS{{O}_{4}}>MgS{{O}_{4}}>BaS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 93) Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions. Ethanal (I), Propanal (II), Propanone (III), Butanone (IV)
A)
III < II < I < IV
done
clear
B)
II < I < III < IV
done
clear
C)
IV < III < II < I
done
clear
D)
I < II < III < IV
done
clear
View Answer play_arrow
question_answer 94) The strongest Lewis acid among boron halides is
A)
\[BB{{r}_{3}}\]
done
clear
B)
\[BC{{l}_{3}}\]
done
clear
C)
\[B{{I}_{3}}\]
done
clear
D)
\[B{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 95) The correct order of increasing hydration energy of the following conjugate bases of oxoacids of chlorine is
A)
\[Cl{{O}^{-}}<ClO_{2}^{-}<ClO_{3}^{-}<ClO_{4}^{-}\]
done
clear
B)
\[ClO_{4}^{-}<ClO_{3}^{-}<ClO_{2}^{-}<Cl{{O}^{-}}\]
done
clear
C)
\[ClO_{4}^{-}<ClO_{3}^{-}<Cl{{O}^{-}}<ClO_{2}^{-}\]
done
clear
D)
\[ClO_{3}^{-}<ClO_{4}^{-}<ClO_{2}^{-}<Cl{{O}^{-}}\]
done
clear
View Answer play_arrow
question_answer 96) Thermodynamically the most stable form of carbon is
A)
diamond
done
clear
B)
graphite
done
clear
C)
fullerenes
done
clear
D)
coal
done
clear
View Answer play_arrow
question_answer 97) The estimation of available chlorine in bleaching powder is done by
A)
acid-base titration
done
clear
B)
permanganometric titration
done
clear
C)
iodimetric titration
done
clear
D)
iodometric titration
done
clear
View Answer play_arrow
question_answer 98) A solid is formed by two elements P and Q. The element Q forms cubic close packing and atoms of P occupy one-third of tetrahedral voids. The formula of the compound is
A)
\[P{{Q}_{3}}\]
done
clear
B)
\[{{P}_{3}}Q\]
done
clear
C)
\[{{P}_{2}}{{Q}_{3}}\]
done
clear
D)
\[{{P}_{3}}{{Q}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) The compound exhibiting maximum value of equivalent conductance in a fused state is
A)
\[SrC{{l}_{2}}\]
done
clear
B)
\[CaC{{l}_{2}}\]
done
clear
C)
\[MgC{{l}_{2}}\]
done
clear
D)
\[BeC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 100) Which of the following have been arranged in decreasing order of oxidation number of sulphur?
A)
\[N{{a}_{2}}{{S}_{4}}{{O}_{6}}>{{H}_{2}}{{S}_{2}}{{O}_{7}}>N{{a}_{2}}{{S}_{2}}{{O}_{3}}>{{S}_{8}}\]
done
clear
B)
\[{{H}_{2}}S{{O}_{4}}>S{{O}_{2}}>{{H}_{2}}S>{{H}_{2}}{{S}_{2}}{{O}_{8}}\]
done
clear
C)
\[SO_{2}^{2+}>SO_{4}^{2-}>SO_{3}^{2-}>HSO_{4}^{-}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{5}}>{{H}_{2}}S{{O}_{3}}>SC{{l}_{2}}>{{H}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 101) Nuclear membrane is absent in
A)
Monera
done
clear
B)
Protista
done
clear
C)
Fungi
done
clear
D)
Plantae
done
clear
View Answer play_arrow
question_answer 102) One of the following is not the characteristic feature of cyanobacteria.
A)
They are multicellular
done
clear
B)
They form colonies
done
clear
C)
They form blooms in polluted water bodies
done
clear
D)
They can fix atmospheric nitrogen
done
clear
View Answer play_arrow
question_answer 103) Heterotrophic fungi can live as
A)
saprophytes
done
clear
B)
symbionts
done
clear
C)
parasites
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 104) In double fertilization
A)
two male gametes ruse with two eggs
done
clear
B)
one male gamete fuses with the egg and the other fuses with the secondary nucleus
done
clear
C)
one male gamete fuses with the egg and the other fuses with the antipodal
done
clear
D)
one male gamete fuses with the antipodal and the other fuses with the diploid nucleus
done
clear
View Answer play_arrow
question_answer 105) Find the correct match.
A)
Mustard plant - Leaves are opposite
done
clear
B)
Mustard plant - Leaves are alternate
done
clear
C)
Guava plant - Leaves are alternate
done
clear
D)
Guava plant - Leaves are whorled
done
clear
View Answer play_arrow
question_answer 106) Which of the following is not correctly paired?
A)
Fabaceae - Legume family
done
clear
B)
Solanaceae - Potato family
done
clear
C)
Liliaceae - Sunflower family
done
clear
D)
Brassicaceae - Mustard family
done
clear
View Answer play_arrow
question_answer 107) Find the incorrect statement.
A)
Root hairs are unicellular elongations
done
clear
B)
Root hairs absorb water and minerals
done
clear
C)
Trichomes are unicellular elongations
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 108) Which one of the following is not correct?
A)
Early wood is characterized by large number of xylary elements
done
clear
B)
Early wood is characterised by vessels with wider cavities
done
clear
C)
Late wood is characterised by large number of xylary elements
done
clear
D)
Late wood is characterised by vessels with narrower cavities
done
clear
View Answer play_arrow
question_answer 109) Cells divide and new cells are formed from pre-existing cells. This concept was given by
A)
Matthias Schleiden
done
clear
B)
Theodore Schwann
done
clear
C)
Matthias Schleiden and Theodore Schwann
done
clear
D)
Rudolf Virchow
done
clear
View Answer play_arrow
question_answer 110)
A)
osmosis
done
clear
B)
diffusion
done
clear
C)
passive transport
done
clear
D)
active transport
done
clear
View Answer play_arrow
question_answer 111) Aleuroplasts in a cell stores
A)
starch
done
clear
B)
oil
done
clear
C)
protein
done
clear
D)
nutrients
done
clear
View Answer play_arrow
question_answer 112) Chiasmata formation takes place during
A)
prophase- I
done
clear
B)
metaphase-I
done
clear
C)
anaphase-II
done
clear
D)
telophase- I
done
clear
View Answer play_arrow
question_answer 113) The size of mitochondria in plant cell is
A)
0.1- 10 \[\mu \]m long
done
clear
B)
1.0- 4.0 \[\mu \]m long
done
clear
C)
2.0- 4.0 \[\mu \]m long
done
clear
D)
3.0- 4.0 \[\mu \]m long
done
clear
View Answer play_arrow
question_answer 114) Active transport is characterized by
A)
requires special membrane proteins
done
clear
B)
highly selective
done
clear
C)
requires ATP energy
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 115) Stomatal opening or closing is due to
A)
change in the turgidity of guard cells
done
clear
B)
the inner walls of each guard cells is thick and elastic
done
clear
C)
cellulose microfibrils of guard cells are oriented radially
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 116) The loss of water in \[{{\text{C}}_{\text{4}}}\text{-}\]plants compared to \[{{\text{C}}_{\text{3}}}\text{-}\]plants for the same amount of \[C{{O}_{2}}\]fixed is
A)
half
done
clear
B)
one- third
done
clear
C)
one- fourth
done
clear
D)
double
done
clear
View Answer play_arrow
question_answer 117) Which one of the following element is not remobilised in leaf?
A)
Nitrogen
done
clear
B)
Phosphorus
done
clear
C)
Potassium
done
clear
D)
Calcium
done
clear
View Answer play_arrow
question_answer 118) Micronutrients are needed in amounts equivalent to
A)
8 m mole/kg of dry matter
done
clear
B)
18 m mole/kg of dry matter
done
clear
C)
25 m mole/kg of dry matter
done
clear
D)
30 m mole/kg of dry matter
done
clear
View Answer play_arrow
question_answer 119) Denitrification is carried out by
A)
Nitrosomonas
done
clear
B)
Pseudomonas
done
clear
C)
Nitrobacter
done
clear
D)
Nitrococcus
done
clear
View Answer play_arrow
question_answer 120) The creation of proton gradient across the thylakoid membrane is a result of
A)
decrease in proton number in stroma
done
clear
B)
accumulation of protons in the lumen
done
clear
C)
decrease in the pH in the lumen
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 121) For every \[C{{O}_{2}}\] molecule entering the Calvin cycle, the number of ATP and NADPH required is
A)
2 ATP+2 NADPH
done
clear
B)
2 ATP+3 NADPH
done
clear
C)
3 ATP+2 NADPH
done
clear
D)
3 ATP+3 NADPH
done
clear
View Answer play_arrow
question_answer 122) In citric acid cycle, decarboxylarion occurs when
A)
citric acid converts to \[\alpha\] -ketoglutaric acid
done
clear
B)
succinic acid converts to malic acid
done
clear
C)
malic acid converts to oxaloacetic acid
done
clear
D)
oxaloacetic acid converts to citric acid
done
clear
View Answer play_arrow
question_answer 123) Which one of the following plant function is not controlled by auxins?
A)
Apical dominance
done
clear
B)
Phototropism
done
clear
C)
Growth
done
clear
D)
Photosynthesis
done
clear
View Answer play_arrow
question_answer 124) Find the incorrect match.
A)
Tap root ? Carrot
done
clear
B)
Adventitious root ? Sweet potato
done
clear
C)
Prop root ? Banyan tree
done
clear
D)
Stilt root ? Turnip
done
clear
View Answer play_arrow
question_answer 125) Medullary rays are made up of
A)
parenchymatous cells
done
clear
B)
sclerenchymatous cells
done
clear
C)
tracheids
done
clear
D)
fibres
done
clear
View Answer play_arrow
question_answer 126) Which one of the following is matched incorrectly?
A)
Pinus ? Coralloid roots
done
clear
B)
Sequoia ? Tap roots
done
clear
C)
Cycos ? Unbranched stem
done
clear
D)
Cedrus ? Branched stem
done
clear
View Answer play_arrow
question_answer 127) One of the following is not true for hydrophytes.
A)
Vessels are usually absent
done
clear
B)
Tracheids are absent
done
clear
C)
Cuticle is poorly developed
done
clear
D)
Air chambers are well developed
done
clear
View Answer play_arrow
question_answer 128) A typical dicotyledonous embryo consists of
A)
radicle only
done
clear
B)
embryonal axis only
done
clear
C)
cotyledons only
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 129) When pollen is transferred from anther of a flower to stigma of another flower of the same plant, pollination is referred to as
A)
geitonogamy
done
clear
B)
allogamy
done
clear
C)
xenogamy
done
clear
D)
siphonogamy
done
clear
View Answer play_arrow
question_answer 130) Synergids are
A)
haploid
done
clear
B)
diploid
done
clear
C)
triploid
done
clear
D)
tetraploid
done
clear
View Answer play_arrow
question_answer 131) The residual persistent nucellus is known as
A)
perisperm
done
clear
B)
pericarp
done
clear
C)
integuments
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 132) Phenotypic ratio in plant snapdragon in \[{{F}_{2}},\] is
A)
1 : 1
done
clear
B)
2 : 1
done
clear
C)
3 : 1
done
clear
D)
1 : 2 : 1
done
clear
View Answer play_arrow
question_answer 133) In transcription in eukaryotes, heterogeneous nuclear RNA (hn RNA) is transcribed by
A)
RNA polymerase- I
done
clear
B)
RNA polymerase- II
done
clear
C)
RNA polymerase-III
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 134) Non- proteinaceous enzyme that acts as a catalyst for the formation of peptide bond, is
A)
spliceosome
done
clear
B)
ribozyme
done
clear
C)
RNA polymerase- I
done
clear
D)
RNA polymerase-III
done
clear
View Answer play_arrow
question_answer 135) In lac operon, i-gene codes for
A)
inducer of lac operon
done
clear
B)
represser of lac operon
done
clear
C)
hydrolysis of disaccharide
done
clear
D)
permease
done
clear
View Answer play_arrow
question_answer 136) Which one of the following has dual functions? It codes for methionine and also cacts as initiator codon
A)
AUG
done
clear
B)
AUC
done
clear
C)
ACU
done
clear
D)
ACA
done
clear
View Answer play_arrow
question_answer 137) Methyl guanosine triphosphate is added to the 5 end of hn RNA in a process of
A)
splicing
done
clear
B)
capping
done
clear
C)
tailing
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 138) A typical nucleosome contains
A)
100 bp of DNA helix
done
clear
B)
200 bp of DNA helix
done
clear
C)
300 bp of DNA helix
done
clear
D)
400 bp of DNA helix
done
clear
View Answer play_arrow
question_answer 139) House- keeping proteins occur in
A)
endoplasmic reticulum
done
clear
B)
Golgi complex
done
clear
C)
cytoskeleton
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 140) The purpose of biological treatment of waste water is to
A)
reduce BOD
done
clear
B)
increase BOD
done
clear
C)
reduce sedimentation
done
clear
D)
increase sedimentation
done
clear
View Answer play_arrow
question_answer 141) The main sources of biofertilizers are
A)
bacteria
done
clear
B)
cyanobacteria
done
clear
C)
fungi
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 142) Maize hybrids have been developed for higher amount of
A)
lysine
done
clear
B)
leucine
done
clear
C)
methionine
done
clear
D)
cysteine
done
clear
View Answer play_arrow
question_answer 143) "Grey biotechnology" is referred to
A)
medical process
done
clear
B)
industrial process
done
clear
C)
agricultural process
done
clear
D)
aquatic process
done
clear
View Answer play_arrow
question_answer 144) Salt tolerant transgenic has been developed for
A)
brinjal
done
clear
B)
grape
done
clear
C)
potato
done
clear
D)
tomato
done
clear
View Answer play_arrow
question_answer 145) Insect tolerant gene from Bacillus thurmgiensis is introduced using T, plasmid of
A)
Escherichia coli
done
clear
B)
Haemophilus influenza
done
clear
C)
Agrobacterium tumefaciens
done
clear
D)
Arabidopsis thaliana
done
clear
View Answer play_arrow
question_answer 146) Pyramid of energy is
A)
always upright
done
clear
B)
always inverted
done
clear
C)
may be upright or inverted
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 147) In an ecosystem, the cycling of nutrients is known as
A)
geological cycle
done
clear
B)
chemical cycle
done
clear
C)
geochemical cycle
done
clear
D)
biogeochemical cycle
done
clear
View Answer play_arrow
question_answer 148) Indias share in the global species diversity is about
A)
2 per cent
done
clear
B)
4 per cent
done
clear
C)
6 per cent
done
clear
D)
8 per cent
done
clear
View Answer play_arrow
question_answer 149) The Air (Prevention and Control of Pollution) Act was amended in 1987 to include one of the following as pollutant.
A)
Water
done
clear
B)
Noise
done
clear
C)
Dust
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 150) One of the following is not a green house gas.
A)
Carbon dioxide
done
clear
B)
Methane
done
clear
C)
Ethane
done
clear
D)
\[{{N}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 151) In the resting muscle fibre, tropomyosin partially covers
A)
calcium binding sites on troponm
done
clear
B)
actin binding sites on myosin
done
clear
C)
myosin binding sites on actin
done
clear
D)
calcium binding sites on actin
done
clear
View Answer play_arrow
question_answer 152) he most commonly maintained species of bee by beekeepers is
A)
Apis mellifera
done
clear
B)
Apis dorsata
done
clear
C)
Apis indica
done
clear
D)
Apisflorea
done
clear
View Answer play_arrow
question_answer 153) Which of the following statements about colour blindness is correct?
A)
2% men are red colorblind, 6% are green colorblind
done
clear
B)
6% men are red colorblind, 2% are green colorblind
done
clear
C)
10% men are red colorblind, 5% are green colorblind
done
clear
D)
5% men are red colorblind, 10% are green colorblind
done
clear
View Answer play_arrow
question_answer 154) The product of which of the following organisms has been commercialized as blood cholesterol lowering agent?
A)
Trichoderma polysporum
done
clear
B)
Saccharomyces cerevisiae
done
clear
C)
Aspergillus niger
done
clear
D)
Monascus purpureus
done
clear
View Answer play_arrow
question_answer 155) Stereocilia occur in
A)
pseudostratified columnar epithelium of trachea
done
clear
B)
columnar epithelium of stomach
done
clear
C)
stratified columnar epithelium of pharynx
done
clear
D)
pseudostratified columnar epithelium of epididymis
done
clear
View Answer play_arrow
question_answer 156) An evolutionary pattern characterized by a rapid increase in the number and kinds of closely related species is called
A)
convergent evolution
done
clear
B)
divergent evolution
done
clear
C)
adaptive radiation
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 157) The lateral hearts in earthworm have
A)
four pairs of valves and are situated in segments 7 and 9
done
clear
B)
four pairs of valves and are situated in segments 6 and 8
done
clear
C)
three pairs of valves and are situated in segments 8 and 10
done
clear
D)
two pairs of valves and are situated in segments 6 and 11
done
clear
View Answer play_arrow
question_answer 158) Which of the following is an exclusively echinoderm character?
A)
Radial symmetry
done
clear
B)
Tube feet
done
clear
C)
Mesodermal endoskeleton
done
clear
D)
Coelom divided
done
clear
View Answer play_arrow
question_answer 159) In the clotting mechanism pathway, thrombin activates factors
A)
XI, VIII, V
done
clear
B)
XI, IX, X
done
clear
C)
VIII, X, V
done
clear
D)
IX, VIII, X
done
clear
View Answer play_arrow
question_answer 160) In myasthenia gravis, acetylcholine
A)
receptors on motor end plate are reduced
done
clear
B)
secretion from nerve terminals is reduced
done
clear
C)
esterase activity is inhibited
done
clear
D)
secretion from nerve terminals is enhanced
done
clear
View Answer play_arrow
question_answer 161) This trace element is needed for insulin to exert its maximal effect in glucose uptake.
A)
Vanadium
done
clear
B)
Chromium
done
clear
C)
Molybdenum
done
clear
D)
Selenium
done
clear
View Answer play_arrow
question_answer 162) Immunoglobulins are proteins that show...... structure.
A)
primary
done
clear
B)
secondary
done
clear
C)
tertiary
done
clear
D)
quaternary
done
clear
View Answer play_arrow
question_answer 163) The cell membranes of adjacent cells are fused at this cell junction.
A)
Macula adherens
done
clear
B)
Zonula adherens
done
clear
C)
Zonula occludens
done
clear
D)
Nexus
done
clear
View Answer play_arrow
question_answer 164) Recently attempts are being made to reintroduce tigers in this famous National Park.
A)
Corbett
done
clear
B)
Bandhavgarh
done
clear
C)
Sariska
done
clear
D)
Kanha
done
clear
View Answer play_arrow
question_answer 165) The phenomenon of torsion occurs in
A)
Gastropoda
done
clear
B)
Pelecypoda
done
clear
C)
Cephalopoda
done
clear
D)
Amphineura
done
clear
View Answer play_arrow
question_answer 166) In humans, the oocyte is maintained in a state of meiotic arrest by secretions of
A)
granulosa cells
done
clear
B)
zona pellucida
done
clear
C)
cumulus oophorus
done
clear
D)
theca
done
clear
View Answer play_arrow
question_answer 167) Animals that rely on the heat from the environment, rather than of metabolism, to raise their body temperature are in the strict sense, called
A)
ectothermic
done
clear
B)
poikilothermic
done
clear
C)
homeothermic
done
clear
D)
endothermic
done
clear
View Answer play_arrow
question_answer 168) During the past 150 years, the concentration of \[C{{O}_{2}}\]has increased approximately from
A)
200 ppm to 300 ppm
done
clear
B)
120 ppm to 280 ppm
done
clear
C)
280 ppm to 370 ppm
done
clear
D)
350 ppm to 450 ppm
done
clear
View Answer play_arrow
question_answer 169) Which of the following sequences will be produced as a result of transcription of the DNA sequence CGATTACAG?
A)
GCUAAUGUC
done
clear
B)
CGUAAUCUG
done
clear
C)
GCTAATGTC
done
clear
D)
GCUAATCTG
done
clear
View Answer play_arrow
question_answer 170) Which statement is incorrect?
A)
Mast cells and basophils secrete histamine and heparin
done
clear
B)
Mast cells are long lived, basophils are short lived
done
clear
C)
Mast cells are smaller than basophils with a bilobed nucleus
done
clear
D)
Mast cells are relatively sessile, basophils are mobile
done
clear
View Answer play_arrow
question_answer 171) Between breaths, the intra pleural pressure is approximately ...... mm Hg less than atmospheric pressure.
A)
1
done
clear
B)
4
done
clear
C)
8
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 172) Microscopic aquatic organisms lacking locomotory ability and drifting with the water currents are
A)
planktons
done
clear
B)
nektons
done
clear
C)
pleustons
done
clear
D)
sestons
done
clear
View Answer play_arrow
question_answer 173) The vestiges of girdles are found in
A)
cobra
done
clear
B)
krait
done
clear
C)
rattle snake
done
clear
D)
python
done
clear
View Answer play_arrow
question_answer 174) Which is a 32-amino acid water soluble peptide hormone?
A)
Gastrin
done
clear
B)
Calcitonin
done
clear
C)
Glucagon
done
clear
D)
Insulin
done
clear
View Answer play_arrow
question_answer 175) Essential and non-essential amino acid is
A)
lysine and leucine
done
clear
B)
methionine and threonine
done
clear
C)
valine and tyrosine
done
clear
D)
alanine and cystine
done
clear
View Answer play_arrow
question_answer 176) Maintenance of body potassium levels is primarily by tubular
A)
absorption in PCT
done
clear
B)
secretion in DCT and cortical collecting duct
done
clear
C)
absorption in DCT
done
clear
D)
secretion in PCT
done
clear
View Answer play_arrow
question_answer 177) In ecological succession, the climax community is best recognized by the following state.
A)
P = R
done
clear
B)
P > R
done
clear
C)
P < R
done
clear
D)
P\[\ne \]R
done
clear
View Answer play_arrow
question_answer 178) The third ventricle of the brain is situated in the
A)
base of telencephalon
done
clear
B)
roof of metencephalon
done
clear
C)
roof of diencephalon
done
clear
D)
base of myelencephalon
done
clear
View Answer play_arrow
question_answer 179) The vibrations of the tympanic membrane are amplified approximately...... times in the oval window.
A)
5
done
clear
B)
20
done
clear
C)
40
done
clear
D)
55
done
clear
View Answer play_arrow
question_answer 180) The energy content in kcal/g of carbohydrate, protein and triglycerol respectively is approximately in the ratio of
A)
1 : 2 : 2
done
clear
B)
1 : 1 : 2
done
clear
C)
2 : 1 : 1
done
clear
D)
2 : 2 : 1
done
clear
View Answer play_arrow
question_answer 181) Originating in bone marrow, circulating in blood for 1 to 2 days, migrating to connective tissue and forming macrophages is a characteristic of
A)
eosinophils
done
clear
B)
basophils
done
clear
C)
monocytes
done
clear
D)
lymphocytes
done
clear
View Answer play_arrow
question_answer 182) In a polluted environment, the maximum pollutant will occur in
A)
primary producers
done
clear
B)
tertiary consumers
done
clear
C)
secondary consumers
done
clear
D)
primary consumers
done
clear
View Answer play_arrow
question_answer 183) The optic lobes in humans are represented by the corpora
A)
bigemina
done
clear
B)
arenacea
done
clear
C)
striata
done
clear
D)
quadrigemina
done
clear
View Answer play_arrow
question_answer 184) Parathormone influences calcium absorption in the small intestine by regulating the metabolism of
A)
vitamin- C
done
clear
B)
vitamin- D
done
clear
C)
vitamin-\[{{B}_{6}}\]
done
clear
D)
enterogasterone
done
clear
View Answer play_arrow
question_answer 185) Which of the following is not an effect of the sympathetic nervous system?
A)
Dilation of pupil
done
clear
B)
Inhibition of peristalsis
done
clear
C)
Elevation of blood pressure
done
clear
D)
Stimulation for saliva secretion
done
clear
View Answer play_arrow
question_answer 186) RNA polymerase- II is responsible for transcription of
A)
rRNA
done
clear
B)
hnRNA
done
clear
C)
cRNA
done
clear
D)
snRNA
done
clear
View Answer play_arrow
question_answer 187) Disappearance of the tadpole tail during metamorphosis, is brought about by
A)
endoplasmic reticulum
done
clear
B)
Golgi bodies
done
clear
C)
lysosomes
done
clear
D)
peroxisomes
done
clear
View Answer play_arrow
question_answer 188) The forward stereoscopic visual field will be the greatest in
A)
cat
done
clear
B)
deer
done
clear
C)
rabbit
done
clear
D)
horse
done
clear
View Answer play_arrow
question_answer 189) This gastrointestinal hormone stimulates insulin secretion.
A)
Gastrin
done
clear
B)
CCK
done
clear
C)
Secretin
done
clear
D)
GIP
done
clear
View Answer play_arrow
question_answer 190) The volume and surface area of a deer is 1,50,000 cm3 and 19,000 cm2 and of a squirrel is 625 cm3 and 530 cm2. The area available for heat loss per cm3 volume of the squirrel will be approximately
A)
seven times more than the deer
done
clear
B)
five times less than the deer
done
clear
C)
three times more than the deer
done
clear
D)
eleven times more than the deer
done
clear
View Answer play_arrow
question_answer 191) The loss of energy as one proceeds from one trophic level to the next higher level is approximately
A)
30 per cent
done
clear
B)
40 per cent
done
clear
C)
60 per cent
done
clear
D)
90 per cent
done
clear
View Answer play_arrow
question_answer 192) This is not a nitrogenous waste.
A)
Creatinine
done
clear
B)
Purine
done
clear
C)
Allantoin
done
clear
D)
Citrulline
done
clear
View Answer play_arrow
question_answer 193) Immediate hypersensitivity which result in the release of histamine and other inflammatory substances is mediated by
A)
IgA
done
clear
B)
IgD
done
clear
C)
IgE
done
clear
D)
IgG
done
clear
View Answer play_arrow
question_answer 194) The direction of light striking the retina will be
A)
photosensory cells \[\to \] bipolar neurons \[\to \] ganglionic cells \[\to \] sensory nerves
done
clear
B)
sensory nerves \[\to \] bipolar neurons \[\to \]ganglionic cells\[\to \]photosensory cells
done
clear
C)
sensory nerves\[\to \]ganglionic cells\[\to \]bipolar neurons\[\to \]photosensory cells
done
clear
D)
photosensory cells\[\to \]ganglionic cells\[\to \] bipolar neurons \[\to \] sensory nerves
done
clear
View Answer play_arrow
question_answer 195) In the mouth parts of cockroach, the galea and lacinia form parts of the
A)
mandible
done
clear
B)
maxilla
done
clear
C)
labium
done
clear
D)
labrum
done
clear
View Answer play_arrow
question_answer 196) In a disaccharide, two monosaccharides are linked by a ... bond.
A)
hydrogen
done
clear
B)
disulphide
done
clear
C)
glycosidic
done
clear
D)
ionic
done
clear
View Answer play_arrow
question_answer 197) In human beings, the cranium is formed by
A)
eight bones of which two are paired
done
clear
B)
fourteen bones of which six are paired
done
clear
C)
ten bones of which two are paired
done
clear
D)
twelve bones of which four are paired
done
clear
View Answer play_arrow
question_answer 198) This sensory structure responds to pressure change.
A)
Meissners corpuscle
done
clear
B)
Pacinian corpuscle
done
clear
C)
End bulb of Krause
done
clear
D)
Organ of Ruffini
done
clear
View Answer play_arrow
question_answer 199) Which statements is true about the venous blood vessels of frog?
A)
Lingual and submandibular unite to form the internal jugular
done
clear
B)
Musculo-cutaneous and brachial unite to form the subclavian
done
clear
C)
The ventral abdominal vein drains into the posterior vena cava
done
clear
D)
The pelvic veins unite to form the renal portal vein
done
clear
View Answer play_arrow
question_answer 200) This is the common passage for bile and pancreatic juices.
A)
Ampulla of Vater
done
clear
B)
Ductus Choledochus
done
clear
C)
Duct of Wirsung
done
clear
D)
Duct of Santorini
done
clear
View Answer play_arrow
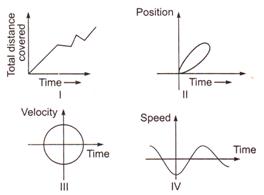 I and II II and III II and IV All four
I and II II and III II and IV All four
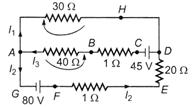
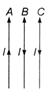
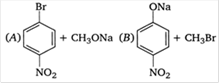
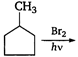 the major product obtained is
the major product obtained is