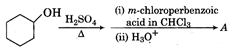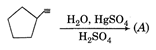question_answer 1) The measured value of length of a simple pendulum is 20 cm known with 2 mm accuracy. The time for 50 oscillations was measured to be 40 s with Is resolution. Calculate, the percentage accuracy in the determination of acceleration due to gravity g from the above measurements.
A)
6.0%
done
clear
B)
7.2%
done
clear
C)
9.4%
done
clear
D)
10.2%
done
clear
View Answer play_arrow
question_answer 2)
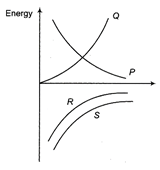
Which of the following curves represents the variation of total energy with radius r for a satellite in a circular orbit?
A)
P
done
clear
B)
Q
done
clear
C)
R
done
clear
D)
S
done
clear
View Answer play_arrow
question_answer 3) In a container, 200 g of aluminum (specific heat 900 J/kg. K) at \[{{100}^{o}}C\] is mixed with 50 g of water at \[{{20}^{o}}C\], with the mixture thermally isolated. Find the entropy change of the aluminum-water system.
A)
+2.8 J/K
done
clear
B)
-22.1 J/K
done
clear
C)
+24.9 J/K
done
clear
D)
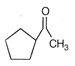
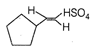
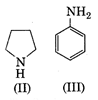
None of the above
done
clear
View Answer play_arrow
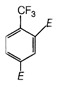
question_answer 4)
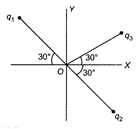
Figure shows three particles with charges\[{{q}_{1}}=2Q,\,{{q}_{2}}=-2Q\] and \[{{q}_{3}}=-4Q\], each a distance d from the origin. Find the net electric field E at the origin.
A)
\[\frac{2.56Q}{4\pi {{\varepsilon }_{0}}{{d}^{2}}}\] towards + ve x-axis
done
clear
B)
\[\frac{6.93Q}{4\pi {{\varepsilon }_{0}}{{d}^{2}}}\] towards + ve x-axis
done
clear
C)
\[\frac{6.93Q}{4\pi {{\varepsilon }_{0}}{{d}^{2}}}\] towards - ve x-axis
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 5) In Millikans experiment, an oil drop of radius 1.64 am and density \[0.85\,\,g/c{{m}^{3}}\] is suspended when a downward electric field of \[1.9\times {{10}^{5}}\,N/C\] is applied. What is the charge on the drop in terms of e?
A)
-9e
done
clear
B)
-7e
done
clear
C)
-5e
done
clear
D)
-3e
done
clear
View Answer play_arrow
question_answer 6) A uniformly charged conducting sphere of diameter 1.2 m has a surface charge density of\[8.1\,\mu C/{{m}^{2}}\]. Find the total electric flux leaving the surface of the sphere.
A)
\[4.1\times {{10}^{6}}N-{{m}^{2}}/C\]
done
clear
B)
\[1.3\times {{10}^{4}}N-{{m}^{2}}/C\]
done
clear
C)
\[-4.1\times {{10}^{6}}N-{{m}^{2}}/C\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 7) A thin non-conducting rod of length 50 cm has a positive charge of uniform linear density\[{{10}^{-12}}C/m\]. Find the electric potential due to the rod at a point, which is at a perpendicular distance of 1.0 cm from one-end of the rod\[({{\varepsilon }_{0}}=8.8\times {{10}^{-12}}F/m)\].
A)
0.02 V
done
clear
B)
0.04 V
done
clear
C)
0.06 V
done
clear
D)
1.02 V
done
clear
E)
None of the above
done
clear
View Answer play_arrow
question_answer 8) Arrange the following materials in the increasing order of their resistivity. Copper, Platinum, Silver, Aluminium
A)
Copper, Silver, Platinum, Aluminium
done
clear
B)
Silver, Copper, Aluminium, Platinum
done
clear
C)
Aluminium, Platinum, Silver, Copper
done
clear
D)
Platinum, Aluminium, Copper, Silver
done
clear
View Answer play_arrow
question_answer 9)
In a given circuit, each cell has an emf of 0.15 V and internal resistance of 0.25 \[\Omega \]. Find the current in the circuit.
A)
0.12 A
done
clear
B)
0.012 A
done
clear
C)
0.045 A
done
clear
D)
0.45 A
done
clear
View Answer play_arrow
question_answer 10)
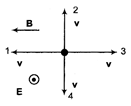
The figure shows four directions for the velocity vector v of a positively charged particle moving through a uniform electric field E and a uniform magnetic field B. Of all four directions, which results in a net force of zero?
A)
4
done
clear
B)
1
done
clear
C)
3
done
clear
D)
2
done
clear
View Answer play_arrow
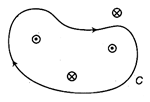
question_answer 11)
Four conductors carrying 2.0 A of current into or out of the page are shown in the diagram. A path C is indicated for the line integral \[\oint{B.\,ds}\] . Find the value of the integral for the path C.
A)
\[2{{\mu }_{0}}\]
done
clear
B)
Zero
done
clear
C)
\[-2{{\mu }_{0}}\]
done
clear
D)
\[-8{{\mu }_{0}}\]
done
clear
View Answer play_arrow
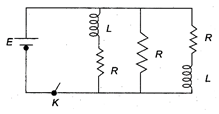
question_answer 12)
Figure shows a circuit that contains three identical resistors with resistance \[R=9.0\,\Omega \], two identical inductors with inductance L = 4.0 mH, and a battery with emf E = 18 V. Find the ratio of the currents in the circuit just after and long after the switch K is closed.
A)
1/3
done
clear
B)
2/3
done
clear
C)
1
done
clear
D)
4/3
done
clear
View Answer play_arrow
question_answer 13) An iron rod is subjected to the cycles of magnetisation at the rate of 50 Hz. Given the density of the rod is 8 x 103 kg/m3 and specific heat is \[0.11\times {{10}^{-3}}\] \[cal/k{{g}^{o}}C\]. The rise in temperature per minute, if the area enclosed by the B-H loop corresponds to energy of \[{{10}^{-2}}J\] is
A)
\[{{78}^{o}}C\]
done
clear
B)
\[{{88}^{o}}C\]
done
clear
C)
\[8.1\,C\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 14) A series L-C-R circuit has inductance L = 12 mH, capacitance \[C=1.2\mu F\], and resistance R = 12\[\Omega \]. At what time will the amplitude of the charge oscillations in the circuit be 10% of its initial value?
A)
2.0 ms
done
clear
B)
3.0 ms
done
clear
C)
4.0 ms
done
clear
D)
5.0 ms
done
clear
E)
(e) None of the above
done
clear
View Answer play_arrow
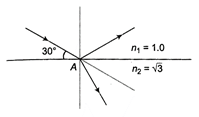
question_answer 15)
A beam of monochromatic light reflects and refracts at point A, as shown in the diagram. Find the angle of refraction at point A.
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{30}^{o}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 16) The electric field of a certain plane electromagnetic wave is given by \[{{E}_{x}}=0\], \[{{E}_{y}}=0,\,{{E}_{z}}=2.0\cos \,[\pi \times {{10}^{15}}(t-x/c)]\] . The wave is propagating in the positive x-direction. Find the expressions for the components of the magnetic field of the wave.
A)
\[{{B}_{x}}=0,\,{{B}_{y}}=6.7\times {{10}^{-9}}\cos [\pi \times {{10}^{16}}(t-x/c)]\],\[{{B}_{z}}=0\]
done
clear
B)
\[{{B}_{x}}=0,\,{{B}_{y}}=-6.7\times {{10}^{-9}}\cos [\pi \times {{10}^{15}}(t-x/c)]\],\[{{B}_{z}}=0\]
done
clear
C)
\[{{B}_{x}}=0,\,{{B}_{y}}=0,\,{{B}_{z}}=2.0\cos [\pi \times {{10}^{15}}(t-x/c)]\]
done
clear
D)
\[{{B}_{x}}=0,\,{{B}_{y}}=0,\,{{B}_{z}}=-6.7\times {{10}^{-9}}\cos [\pi (t-x/c)]\]
done
clear
View Answer play_arrow
question_answer 17) A beam of parallel light rays is incident on a solid transparent sphere of refractive index \[\mu \]. If a point image is produced just at the back of the sphere, what is the refractive index of the sphere?
A)
2.0
done
clear
B)
2.3
done
clear
C)
2.5
done
clear
D)
2.7
done
clear
View Answer play_arrow
question_answer 18) In a double slit experiment, the distance between slits is 5.0 mm and the slits are 1.0 m from the screen. Two interference patterns can be seen on the screen; one due to light with wavelength 480 nm and the other due to light with wavelength 600 nm. Find the separation on the screen between the third order bright fringes of the two interference patterns.
A)
0.072 mm
done
clear
B)
0.063 mm
done
clear
C)
0.037 mm
done
clear
D)
0.019 mm
done
clear
View Answer play_arrow
question_answer 19) Find the de-Broglie wavelength of an electron with a kinetic energy of 120 eV.
A)
95pm
done
clear
B)
102pm
done
clear
C)
112pm
done
clear
D)
124pm
done
clear
View Answer play_arrow
question_answer 20) A LED is constructed from a p-n junction based on a certain semi-conducting material whose energy gap is 1.9 eV. Find the wavelength of the emitted light.
A)
\[2.9\times {{10}^{-9}}m\]
done
clear
B)
\[1.6\times {{10}^{-8}}m\]
done
clear
C)
\[6.5\times {{10}^{-7}}m\]
done
clear
D)
\[9.1\times {{10}^{-5}}m\]
done
clear
View Answer play_arrow
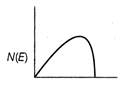
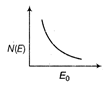
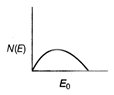
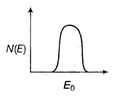
question_answer 21) The energy spectrum of \[\beta \]-particle [number N(E,) as a function of \[\beta \]-energy E] emitted from a radioactive source is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 22) For the following fission reaction\[^{235}U+n{{\xrightarrow{{}}}^{140}}Ce{{+}^{94}}Zr+2n\],Find the disintegration energy. \[({{M}_{U}}=23502\,u,\,{{M}_{n}}=1.0\,u,\,\,{{M}_{Ce}}=139.9\,\,u\],\[{{M}_{Zr}}=93.9\,\,u)\]
A)
205 MeV
done
clear
B)
198 MeV
done
clear
C)
123 MeV
done
clear
D)
89 MeV
done
clear
View Answer play_arrow
question_answer 23) Find the minimum frequency of a photon that can produce a singly ionized He-atom.
A)
\[5.6\times {{10}^{10}}Hz\]
done
clear
B)
\[6.9\times {{10}^{12}}Hz\]
done
clear
C)
\[7.3\times {{10}^{13}}Hz\]
done
clear
D)
\[1.31\,\,\,{{10}^{15}}Hz\]
done
clear
View Answer play_arrow
question_answer 24) The work functions of four materials \[{{M}_{1}},{{M}_{2}},{{M}_{3}}\] and \[{{M}_{4}}\] are 1.9 eV, 2.5 eV, 3.6 eV, and 4.2 eV, respectively. Which of these material is (are) useful in a photocell to detect visible light?
A)
\[{{M}_{1}}\]only
done
clear
B)
\[{{M}_{1}}\] and \[{{M}_{2}}\]
done
clear
C)
\[{{M}_{3}}\] only
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 25) Two amplifiers are connected in series. The first amplifier has a voltage gain of 10 while the second one has voltage gain of 20. If the input signal is 0.01 V, calculate the voltage of the output AC signal.
A)
2V
done
clear
B)
3V
done
clear
C)
4V
done
clear
D)
5V
done
clear
View Answer play_arrow
question_answer 26) Amplitude modulation has
A)
one carrier with two side band frequencies
done
clear
B)
one carrier
done
clear
C)
one carrier with infinite frequencies
done
clear
D)
one carrier with high frequency
done
clear
View Answer play_arrow
question_answer 27) Calculate the focal length of reading glasses of a person, if his distance of distinct vision is 75 cm.
A)
25.6 cm
done
clear
B)
37.5 cm
done
clear
C)
75.2 cm
done
clear
D)
100.4 cm
done
clear
View Answer play_arrow
question_answer 28)
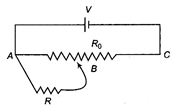
A battery of V voltage is connected across the potentiometer wire AC of total resistance \[{{R}_{0}}\]as shown. Calculate, the potential across the resistance R, if the sliding contact point B is exactly at the middle of the potentiometer wire.
A)
\[\frac{2VR}{4{{R}_{0}}+R}\]
done
clear
B)
\[\frac{4VR}{2{{R}_{0}}+R}\]
done
clear
C)
\[\frac{2VR}{{{R}_{0}}+4R}\]
done
clear
D)
\[\frac{4V{{R}_{0}}}{{{R}_{0}}+2R}\]
done
clear
View Answer play_arrow
question_answer 29) The diameter of a given wire is measured by a screw gauze. The three measurements of the diameter give the reading in cm as 0.036, 0.035 and 0.037. What is the percentage error of the measurement?
A)
1.8%
done
clear
B)
2.8%
done
clear
C)
3.2%
done
clear
D)
4.6%
done
clear
View Answer play_arrow
question_answer 30) Which of the following ratios has the dimension of mass?
A)
Volume / Density
done
clear
B)
Surface tension/\[{{\left( Angular\text{ }velocity \right)}^{2}}\]
done
clear
C)
Linear momentum / Force
done
clear
D)
Pressure / Power
done
clear
View Answer play_arrow
question_answer 31) The distance x of a particle moving in one I dimension under the action of constant force is related to the time t by the relation,\[t=\sqrt{x}+3\]Find the displacement of the particle when its velocity is 6.0 m/s.
A)
9.0m
done
clear
B)
6.0m
done
clear
C)
4.0 m
done
clear
D)
0.0 m
done
clear
View Answer play_arrow
question_answer 32) Which of the following pairs of vectors are parallel?
A)
\[A=\hat{i}-2\hat{j}\,;\,B=\hat{i}-5\hat{j}\]
done
clear
B)
\[A=\hat{i}-10\hat{j}\,;\,B=2\hat{i}-5\hat{j}\]
done
clear
C)
\[A=\hat{i}-5\hat{j}\,;\,B=\hat{i}-10\hat{j}\]
done
clear
D)
\[A=\hat{i}-5\hat{j}\,;\,B=2\hat{i}-10\hat{j}\]
done
clear
View Answer play_arrow
question_answer 33) From the top of a tower of height 10 m, one fire is shot horizontally with a speed of \[5\sqrt{3}\]m/s. Another fire is shot upwards at angle of \[{{60}^{o}}\] with the horizontal at some interval of time with the same speed of \[5\sqrt{3}\] m/s. The shots collide in air at a certain point. The time interval between the two fires is
A)
2s
done
clear
B)
1 s
done
clear
C)
0.5 s
done
clear
D)
0.25 s
done
clear
View Answer play_arrow
question_answer 34) A tube of length L is filled completely with an incompressible liquid of mass M and closed at both ends. The tube is rotated in a horizontal plane about one of its ends with a uniform angular velocity co. The force exerted by the liquid at the other end is.
A)
\[\frac{1}{2}M{{\omega }^{2}}L\]
done
clear
B)
\[M{{\omega }^{2}}L\]
done
clear
C)
\[\frac{1}{4}M{{\omega }^{2}}L\]
done
clear
D)
\[\frac{1}{6}M{{\omega }^{2}}L\]
done
clear
View Answer play_arrow
question_answer 35) In an elastic collision, a neutron collides with carbon. How much energy (in percentage) of neutron is transferred to carbon?
A)
90%
done
clear
B)
45%
done
clear
C)
28 %
done
clear
D)
12%
done
clear
View Answer play_arrow
question_answer 36) A block of mass 1.0 kg moving on a horizontal surface with speed 2m/s enters a rough surface. The retarding force \[({{F}_{r}})\] on the block is given by \[{{F}_{r}}=-\frac{k}{x};\,10\,m<x<100\,m\]\[=0\,;\,x<10\,m\] and \[x>100\,\,m\],where, \[k=0.5\,J\]. The kinetic energy of the block at \[x=100\,m\] is
A)
4.5 J
done
clear
B)
2.5 J
done
clear
C)
0.5 J
done
clear
D)
1.5 J
done
clear
View Answer play_arrow
question_answer 37)
Two uniform rods of different materials \[{{M}_{1}}\] and \[{{M}_{2}}\] have lengths 2 m and 3 m, respectively. The mass per unit length of rods \[{{M}_{1}}\] and \[{{M}_{2}}\] are 1 kg and 2 kg respectively. If the rods are arranged, as shown, the position of the cm relative to point O is
A)
4.9m
done
clear
B)
3.9 m
done
clear
C)
2.9 m
done
clear
D)
2.2 m
done
clear
View Answer play_arrow
question_answer 38) If we consider the mass of black hole as the mass of the earth \[({{M}_{e}})\], then the radius of black hole would be
A)
\[\frac{2G{{M}_{e}}}{{{C}^{2}}}\]
done
clear
B)
\[\frac{2G{{M}_{e}}}{3{{C}^{2}}}\]
done
clear
C)
\[\frac{G{{M}_{e}}}{3{{C}^{2}}}\]
done
clear
D)
\[\frac{G{{M}_{e}}}{{{C}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 39) The surface tension and vapour pressure of water at \[{{30}^{o}}C\] are \[7.2\times {{10}^{-2}}\] N/m and\[2.4\times {{10}^{3}}\], respectively. What is the radius of the smallest droplet of water which can be formed without evaporating at \[{{30}^{o}}C\]?
A)
\[1.6\times {{10}^{-2}}m\]
done
clear
B)
\[2.9\times {{10}^{-3}}m\]
done
clear
C)
\[5.6\times {{10}^{-4}}m\]
done
clear
D)
\[6.0\times {{10}^{-5}}m\]
done
clear
View Answer play_arrow
question_answer 40) The temperature on a Fahrenheit scale is\[98.6{{\,}^{o}}F\]? What is the corresponding temperature on a kelvin scale?
A)
310.2 K
done
clear
B)
280.3 K
done
clear
C)
420.5 K
done
clear
D)
370.6 K
done
clear
View Answer play_arrow
question_answer 41) Two spherical bodies P (radius 9 cm) and Q (radius 27 cm) are at temperatures \[{{T}_{p}}\] and \[{{T}_{Q}}\]respectively. If the maximum intensities in the emission spectra of P and Q are, respectively, at 300 nm and 900 nm, what is the ratio of the rate of energy radiated by P to that by Q?
A)
6
done
clear
B)
7
done
clear
C)
8
done
clear
D)
9
done
clear
View Answer play_arrow
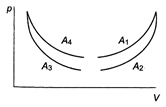
question_answer 42)
The four curves \[{{A}_{1}},{{A}_{2}},{{A}_{3}}\] and \[{{A}_{4}}\] are shown on p-V diagram. Which of the curves represents adiabatic process?
A)
\[{{A}_{3}}\]
done
clear
B)
\[{{A}_{4}}\]
done
clear
C)
\[{{A}_{1}}\]
done
clear
D)
\[{{A}_{2}}\]
done
clear
View Answer play_arrow
question_answer 43) The mean free path of a gas molecule at STP is\[2.1\times {{10}^{-7}}m\]. Find the diameter of the molecule. (Boltzmann constant\[=1.4\times {{10}^{23}}J/K\])
A)
\[5.2\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[4.6\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[3.5\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[2.1\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 44) For a damped harmonic oscillator of mass 200 g, the values of spring constant and damping constant are, respectively, 90 N/m and 0.04 kg/s. The time taken for its amplitude of vibration of drop to half of its initial value is (log, 2 = 0.693)
A)
7.0s
done
clear
B)
14.2 s
done
clear
C)
15.9s
done
clear
D)
26.6 s
done
clear
View Answer play_arrow
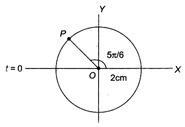
question_answer 45)
For the following reference circle, the equation of simple harmonic motion (SHM) is
A)
\[x=-2\sin (2\pi t+\pi /4)\]
done
clear
B)
\[x=-2\sin (3t+\pi /3)\]
done
clear
C)
\[x=-2\cos (\pi /6-t)\]
done
clear
D)
\[x=-2\cos \pi t\]
done
clear
View Answer play_arrow
question_answer 46) Find the equation of a plane progressive wave travelling along positive: c-axis having amplitude 0.04m, frequency 120 Hz and speed 360 m/s.
A)
\[y=0.04\sin 2\pi (120\,t-x/3)\]
done
clear
B)
\[y=-0.04\sin 2\pi (110\,t-x/3)\]
done
clear
C)
\[y=0.04\sin 2\pi (120\,t+x/3)\]
done
clear
D)
\[y=0.04\sin \,\pi (120\,t-x/3)\]
done
clear
View Answer play_arrow
question_answer 47) A 70 cm long sonometer wire is in unison with a tuning fork. If the length of the wire is decreased by 1.0 cm, it produces 4 beats per second with the same tuning fork. The frequency of the tuning fork is
A)
186 Hz
done
clear
B)
220 Hz
done
clear
C)
276 Hz
done
clear
D)
312 Hz
done
clear
View Answer play_arrow
question_answer 48) Two open organ pipes on sounding together produce 5 beats per second. If the length of the smaller pipe is 0.66m, the length of the larger pipe would be
A)
0.95m
done
clear
B)
0.85 m
done
clear
C)
0.75 m
done
clear
D)
0.67 m
done
clear
View Answer play_arrow
question_answer 49) An observer moves towards a stationary source of sound with a velocity one-fifth the velocity of sound. What is the percentage change in the apparent frequency?
A)
Increase by 0.5 %
done
clear
B)
Decrease by 5 %
done
clear
C)
Increase by 20 %
done
clear
D)
Increase by 50 %
done
clear
View Answer play_arrow
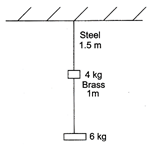
question_answer 50)
A steel wire of 1.5 m long of diameter 0.25 cm and a brass wire of 1.0 m long of diameter 0.25 m are loaded as shown. Calculate, the elongation of the brass wire.
A)
\[1.1\times {{10}^{-4}}m\]
done
clear
B)
\[1.3\times {{10}^{-4}}m\]
done
clear
C)
\[1.5\times {{10}^{-4}}m\]
done
clear
D)
\[1.7\times {{10}^{-4}}m\]
done
clear
View Answer play_arrow
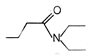
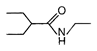
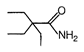
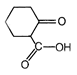
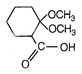
question_answer 51) Which one of the following is an example of \[{{3}^{o}}\] amide?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
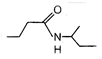
question_answer 52)
End product of the following reaction is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
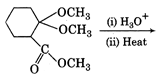
question_answer 53)
The major product of the reaction of
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 54)
End product of the following sequence of reaction is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 55) Which one is least reactive towards nucleophilic substitution reaction?
A)
o-aminochlorobenzene
done
clear
B)
Chlorobenzene
done
clear
C)
o-nitrochlorobenzene
done
clear
D)
2, 4-dinitrochlorobenzene
done
clear
View Answer play_arrow
question_answer 56) A liquid which decomposes at its boiling point can be purified by
A)
distillation at atmospheric pressure
done
clear
B)
distillation under reduced pressure
done
clear
C)
fractional distillation
done
clear
D)
steam distillation
done
clear
View Answer play_arrow
question_answer 57) Which of the following compounds undergoes nitration more readily?
A)
Benzene
done
clear
B)
Benzoic acid
done
clear
C)
Nitrobenzene
done
clear
D)
Toluene
done
clear
View Answer play_arrow
question_answer 58) Enthalpy of combustion of carbon to \[C{{O}_{2}}\] is -393.5 kJ \[mo{{l}^{-1}}\]. What amount of heat will be released upon formation of 35.2 g of \[C{{O}_{2}}\] from carbon and oxygen gas?
A)
214.8kJ
done
clear
B)
314.8kJ
done
clear
C)
414.8kJ
done
clear
D)
514.8kJ
done
clear
View Answer play_arrow
question_answer 59) One mole of an ideal gas at 300 K is expanded isothermally from initial volume of 1 L to 10 L. The \[\Delta E\] for this process is (R = 2 cal\[mo{{l}^{-1}}{{K}^{-1}}\])
A)
163.7 cal
done
clear
B)
zero
done
clear
C)
138.1 cal
done
clear
D)
9 L atom
done
clear
View Answer play_arrow
question_answer 60) For a certain process, and \[\Delta H=178\,kJ\]\[\Delta S=160\,J/K\]. What is the minimum temperature at which the process is spontaneous (assuming that \[\Delta H\] and \[\Delta S\] do not vary with temperature)
A)
2112.3 K
done
clear
B)
136.7K
done
clear
C)
275.8 K
done
clear
D)
1112.5K
done
clear
View Answer play_arrow
question_answer 61) A person inhales 640 g of \[{{O}_{2}}\] per day. If all \[{{O}_{2}}\]is used for converting sugar into \[C{{O}_{2}}\] and\[{{H}_{2}}O\], how much sucrose \[({{C}_{12}}{{H}_{22}}{{O}_{11}})\] is consumed in the body in one day and what is the heat evolved? \[\Delta H\] for combustion of sucrose \[=-5645\,kJ\,mo{{l}^{-1}}\]
A)
530 g, 9403.34 kJ
done
clear
B)
570 g, 9408.34 kJ
done
clear
C)
500 g, 9402.27 kJ
done
clear
D)
520 g, 9435.21 kJ
done
clear
View Answer play_arrow
question_answer 62) Copper crystallises in a face centred cubic lattice with a unit cell length of 361 pm. What is the radius of copper atom in pm?
A)
157
done
clear
B)
181
done
clear
C)
108
done
clear
D)
128
done
clear
View Answer play_arrow
question_answer 63) For the set of reactions,(i) \[A+BC\,;\] (ii) \[C+B\xrightarrow[{}]{{{k}_{3}}}D,\]\[{{k}_{1}}\,[A][B]-{{k}_{2}}[C]-{{k}_{3}}[C][B]\] is equal to
A)
\[\frac{-d\,[A]}{dt}\]
done
clear
B)
\[\frac{-d\,[B]}{dt}\]
done
clear
C)
\[\frac{d\,[C]}{dt}\]
done
clear
D)
\[\frac{d\,[D]}{dt}\]
done
clear
E)
None of the above
done
clear
View Answer play_arrow
question_answer 64) For the reaction, \[2{{N}_{2}}{{O}_{5}}\xrightarrow{{}}4N{{O}_{2}}+{{O}_{2}};\] the rate is directly proportional to \[[{{N}_{2}}{{O}_{5}}]\]. At\[{{45}^{o}}C\], 90% of the \[{{N}_{2}}{{O}_{5}}\] reacts in 3600 seconds. The value of the rate constant is
A)
\[3.2\times {{10}^{-4}}{{s}^{-1}}\]
done
clear
B)
\[6.4\times {{10}^{-4}}{{s}^{-1}}\]
done
clear
C)
\[8.5\times {{10}^{-4}}{{s}^{-1}}\]
done
clear
D)
\[12.8\times {{10}^{-4}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 65) What happens in a steady state?
A)
Heat is evolved
done
clear
B)
The concentration of an intermediate is constant
done
clear
C)
Product is being formed faster than reactants are regenerated
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 66) Nitrogen gas is physically adsorbed on iron at \[{{190}^{o}}C\] but chemisorbed to form a nitride at
A)
\[{{250}^{o}}C\]
done
clear
B)
\[{{300}^{o}}C\]
done
clear
C)
\[{{500}^{o}}C\]
done
clear
D)
\[{{190}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 67) In the equilibrium, \[{{N}_{2}}{{O}_{4}}(g)2N{{O}_{2}}(g)\]the \[{{N}_{3}}{{O}_{4}}\] is fifty per cent dissociated at \[{{60}^{o}}C\]. What will be the value of \[{{K}_{p}}\] at this temperature and one atmosphere?
A)
0.33 atm
done
clear
B)
1.33 atm
done
clear
C)
2.33 atm
done
clear
D)
3.33 atm
done
clear
View Answer play_arrow
question_answer 68) 28 g of \[{{N}_{2}}\] and 6 g of \[{{H}_{2}}\] were mixed. At equilibrium, 17 g \[N{{H}_{3}}\] was produced. The weights of \[{{N}_{2}}\] and \[{{H}_{2}}\] at equilibrium are respectively
A)
11 g, 0 g
done
clear
B)
1 g, 3 g
done
clear
C)
14 g, 3 g
done
clear
D)
11 g, 3 g
done
clear
View Answer play_arrow
question_answer 69) The equilibrium constant for the reaction\[2G+JD+2T\] is \[1.5\times {{10}^{3}}\]. When 1.0 mole of G, 2.0 mole of J and 0.5 mole of D are put in a 1.0 L flask and allowed to reach equilibrium, then the equilibrium concentration of T is
A)
0.039
done
clear
B)
0.078
done
clear
C)
2
done
clear
D)
5
done
clear
E)
None of the above
done
clear
F)
done
clear
View Answer play_arrow
question_answer 70) The value of the equilibrium constant for the reaction, \[{{N}_{2}}+2{{O}_{2}}2N{{O}_{2}}\] is 100. The equilibrium constant for the reaction \[N{{O}_{2}}\frac{1}{2}{{N}_{2}}+{{O}_{2}}\] will be
A)
100
done
clear
B)
0.010
done
clear
C)
0.10
done
clear
D)
1000
done
clear
View Answer play_arrow
question_answer 71) Predict the effect of increased pressure on the following reaction equilibrium, \[2S{{O}_{2}}(g)+{{O}_{2}}(g)2S{{O}_{3}}(g)\]
A)
Equilibrium shift to the right
done
clear
B)
Equilibrium shift to the left
done
clear
C)
No effect on equilibrium
done
clear
D)
Reaction stops
done
clear
View Answer play_arrow
question_answer 72) What mass of \[{{N}_{2}}{{H}_{4}}\] can be oxidised to \[{{N}_{2}}\] by\[24.0\,g\,{{K}_{2}}C{{r}_{2}}{{O}_{7}}\], which is reduced to \[Cr(OH)_{4}^{-}\]?
A)
194.2 g
done
clear
B)
2.97 g
done
clear
C)
2.40 g
done
clear
D)
32 g
done
clear
E)
None of these above
done
clear
View Answer play_arrow
question_answer 73) Given the reaction for the discharge of a cobalt-cadmium battery \[2Co{{(OH)}_{3}}+Cd+2{{H}_{2}}O\xrightarrow{{}}\]\[2Co{{(OH)}_{2}}+Cd{{(OH)}_{2}}\] What species is oxidised during the discharge of the battery?
A)
\[C{{o}^{3+}}\]
done
clear
B)
\[C{{o}^{2+}}\]
done
clear
C)
Cd
done
clear
D)
\[C{{d}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 74) What is the oxidation number of vanadium in\[R{{b}_{4}}Na[H{{V}_{10}}{{O}_{28}}]\]?
A)
+8
done
clear
B)
+5
done
clear
C)
+3
done
clear
D)
+1
done
clear
View Answer play_arrow
question_answer 75) The compound not acting as reducing agent is
A)
\[S{{O}_{2}}\]
done
clear
B)
\[Se{{O}_{2}}\]
done
clear
C)
\[Te{{O}_{2}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 76) Choose the correct alternative for the compounds: \[[Ni{{(CO)}_{4}}]\]and \[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
A)
Tetrahedral, Paramagnetic Tetrahedral, Diamagnetic
done
clear
B)
Tetrahedral, Diamagnetic Square planar, Diamagnetic
done
clear
C)
Square planar, Diamagnetic Square planar, Paramagnetic
done
clear
D)
Trigonal pyramidal Square pyramidal
done
clear
View Answer play_arrow
question_answer 77) There are no S?S bond in
A)
\[{{S}_{2}}O_{4}^{2-}\]
done
clear
B)
\[{{S}_{2}}O_{5}^{2-}\]
done
clear
C)
\[{{S}_{2}}O_{3}^{2-}\]
done
clear
D)
\[{{S}_{2}}O_{7}^{2-}\]
done
clear
View Answer play_arrow
question_answer 78) The correct order of acid strength
A)
\[C{{l}_{2}}{{O}_{7}}>S{{O}_{2}}>{{P}_{4}}{{O}_{10}}\]
done
clear
B)
\[C{{O}_{2}}>{{N}_{2}}{{O}_{5}}>S{{O}_{3}}\]
done
clear
C)
\[N{{a}_{2}}O>,MgO>A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[{{K}_{2}}O>CaO>MgO\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following compound is expected to be coloured?
A)
\[A{{g}_{2}}S{{O}_{4}}\]
done
clear
B)
\[Cu{{F}_{2}}\]
done
clear
C)
\[Mg{{F}_{2}}\]
done
clear
D)
CuCI
done
clear
View Answer play_arrow
question_answer 80) Which one of the following exhibits the maximum covalent character?
A)
\[FeC{{l}_{2}}\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[MgC{{l}_{2}}\]
done
clear
D)
\[SnC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 81) Which one of the following is paramagnetic? I
A)
NO
done
clear
B)
\[N{{O}^{+}}\]
done
clear
C)
\[N{{O}^{-}}\]
done
clear
D)
\[{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 82) Zr (Z = 40) and Hf (Z = 72) have similar atomic and ionic radii because of
A)
both belong to same group
done
clear
B)
diagonal relationship
done
clear
C)
lanthanide contraction
done
clear
D)
having similar chemical properties
done
clear
View Answer play_arrow
question_answer 83) In a square planar complex of the type [Mabcx], the number of geometrical isomers can be
A)
no geometrical isomer
done
clear
B)
three
done
clear
C)
two
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 84) First noble gas compound prepared by Neil Bartlett is
A)
\[O_{2}^{+}PtF_{6}^{-}\]
done
clear
B)
\[O_{3}^{+}PtF_{6}^{-}\]
done
clear
C)
\[X{{e}^{+}}PtF_{6}^{-}\]
done
clear
D)
\[Xe{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 85) Holmes signal uses chemical compound
A)
calcium carbide
done
clear
B)
calcium phosphide
done
clear
C)
calcium carbide and calcium phosphide
done
clear
D)
calcium carbide and aluminium carbide
done
clear
View Answer play_arrow
question_answer 86) Ultimate product is obtained on heating \[{{B}_{2}}{{H}_{6}}\]with \[N{{H}_{3}}\] is
A)
\[{{B}_{3}}{{N}_{3}}{{H}_{6}}\]
done
clear
B)
\[{{(B-N)}_{3}}\]
done
clear
C)
\[{{N}_{2}}{{H}_{4}}\]
done
clear
D)
\[BH_{4}^{-}\]
done
clear
View Answer play_arrow
question_answer 87) Which is least stable compound?
A)
\[BC{{l}_{3}}\]
done
clear
B)
\[GaC{{l}_{3}}\]
done
clear
C)
\[\ln C{{l}_{3}}\]
done
clear
D)
\[TIC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 88) A commercial sample of \[{{H}_{2}}{{O}_{2}}\] marked as 100 volume hydrogen peroxide, it means that
A)
\[1\,mL\,{{H}_{2}}{{O}_{2}}\] will give \[100\,mL\,{{O}_{2}}\] at STP
done
clear
B)
1 L of \[{{H}_{2}}{{O}_{2}}\] will give \[100\,mL\,{{O}_{2}}\] at STP
done
clear
C)
1 L of \[{{H}_{2}}{{O}_{2}}\] will give 22.4 L \[{{O}_{2}}\] at STP
done
clear
D)
1 mL of \[{{H}_{2}}{{O}_{2}}\] will give 1 mole of \[{{O}_{2}}\] at STP
done
clear
View Answer play_arrow
question_answer 89) Find wrong statement
A)
\[N{{a}_{2}}C{{O}_{3}}\] is used in glass industry
done
clear
B)
\[KHC{{O}_{3}}\] is acidic salt
done
clear
C)
\[{{K}_{2}}C{{O}_{3}}\] can be prepared by Solvay process
done
clear
D)
\[N{{a}_{2}}C{{O}_{3}}\] is used for metal refining
done
clear
View Answer play_arrow
question_answer 90) Which of the following facts about the complex \[[Cr{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\] is wrong?
A)
The complex is paramagnetic
done
clear
B)
The complex is an outer orbital complex
done
clear
C)
The complex gives white precipitate with silver nitrate
done
clear
D)
The complex involves \[{{d}^{2}}s{{p}^{3}}\] hybridisation and is octahedral in shape
done
clear
View Answer play_arrow
question_answer 91)
In the given reaction, the compound A is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 92) What reagent(s) can be used to convert 2 methyl pentan-1-ol into 2-methyl pentanal?
A)
\[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
B)
\[LiAl{{H}_{4}}\]
done
clear
C)
\[Cr{{O}_{3}}\]-pyridine
done
clear
D)
\[KMn{{O}_{4}}\]
done
clear
View Answer play_arrow
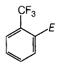
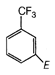
question_answer 93) Which one of the following is most reactive towards nucleophilic substitution reaction?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 94) Which of the following names does not fit a real name?
A)
3-methyl-3-hexanone
done
clear
B)
4-methyl-S-hexanone
done
clear
C)
3-methyl-3-hexanol
done
clear
D)
2-methyl cyclohexanone
done
clear
View Answer play_arrow
question_answer 95) Major product of the reaction is \[{{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}\xrightarrow{ICI}A\]
A)
2-chloro-1-iodo-2-methyl propane
done
clear
B)
1 -chloro-2-iodo-2-methyl propane
done
clear
C)
1, 2-dichloro-2-methyS propane
done
clear
D)
1, 2-diiodo-2-methyl propane
done
clear
View Answer play_arrow
question_answer 96) The source of nitrogen in Gabriel synthesis of primary amines is
A)
potassium cyanide
done
clear
B)
potassium phthaiimide
done
clear
C)
sodium azide
done
clear
D)
sodium nitrite
done
clear
View Answer play_arrow
question_answer 97)
The correct increasing order of basic strength for the following compound is
A)
\[III<I<II\]
done
clear
B)
\[III<II<I\]
done
clear
C)
\[I<II<III\]
done
clear
D)
\[II<I<III\]
done
clear
View Answer play_arrow
question_answer 98) Which of the following reactions involves the formation of a methyl ester from a carboxylic acid?
A)
Hell-Volhard-Zeiinsky reaction
done
clear
B)
Hunsdiecker reaction
done
clear
C)
Reaction with ammonia
done
clear
D)
Reaction with diazomethane
done
clear
View Answer play_arrow
question_answer 99) An ether solution of \[PhC{{H}_{3}}(I),\,PhN{{H}_{2}}(II)\]and \[PhOH(III)\] is extracted with aqueous\[NaOH\]. The ether layer will contain what compound(s) after the extraction?
A)
Only III
done
clear
B)
I + II
done
clear
C)
II + III
done
clear
D)
I + III
done
clear
View Answer play_arrow
question_answer 100)
Final major product of the following reactions is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 101) Nistch was able to get strawberries of different shapes by
A)
splitting the ovary
done
clear
B)
removing the perianth
done
clear
C)
selectively removing some carpels
done
clear
D)
inserting an alcohol dipped needle into the ovary
done
clear
View Answer play_arrow
question_answer 102) Plants can be made disease resistant through
A)
colchicine treatment
done
clear
B)
X-ray treatment
done
clear
C)
breeding with wild relatives
done
clear
D)
hormone treatment
done
clear
View Answer play_arrow
question_answer 103) Yeast is used for commercial production of
A)
methanol
done
clear
B)
ethanol
done
clear
C)
butanol
done
clear
D)
citric acid
done
clear
View Answer play_arrow
question_answer 104) Microbe used as clot buster during myocardial infraction
A)
Penicillium notatum
done
clear
B)
Clostridium butyl icum
done
clear
C)
Streptococcus
done
clear
D)
Acetobacter aceti
done
clear
View Answer play_arrow
question_answer 105) ICBN stands for
A)
Indian Council of British Nature
done
clear
B)
International Code for Biological Nomenclature
done
clear
C)
International Code for Botanical Nomenclature
done
clear
D)
Indian Code for Biological Nomenclature
done
clear
View Answer play_arrow
question_answer 106) Which pair of plants placed in the family- Solanaceae?
A)
Datura and Petunia
done
clear
B)
Datura and Asphodelus
done
clear
C)
Petunia and Sesbania
done
clear
D)
Petunia and Pisum
done
clear
View Answer play_arrow
question_answer 107) Which of the following statements is correct?
A)
Species diversity, in general, increases from poles to the equator
done
clear
B)
Conventional taxonomic methods are equally suitable for higher plants and microorganisms
done
clear
C)
Indias share of global species diversity is about 18%.
done
clear
D)
There are about 25000 known species of plants in India
done
clear
View Answer play_arrow
question_answer 108) PAE stands for
A)
Photosynthetically Adaptable Radiation
done
clear
B)
Photosynthetically Accessible Radiation
done
clear
C)
Photosynthetic Activity Radiometry
done
clear
D)
Photosynthetically Active Radiation
done
clear
View Answer play_arrow
question_answer 109) Which of the following is the largest taxon among plants in terms of the number of species?
A)
Algae
done
clear
B)
Mosses
done
clear
C)
Ferns
done
clear
D)
Fungi
done
clear
View Answer play_arrow
question_answer 110) Which of the following processes will be most adversely affected if microorganisms are removed from a forest ecosystem?
A)
Solar energy fixation and nutrient cycling
done
clear
B)
Decomposition of organic matter and photosynthesis
done
clear
C)
Nitrogen-fixation and decomposition of organic matter
done
clear
D)
Carbon assimilation and nitrogen-fixation
done
clear
View Answer play_arrow
question_answer 111) Residual, persistent nucellus present in some seeds is known as
A)
scutellum
done
clear
B)
perisperm
done
clear
C)
tapetum
done
clear
D)
coleoptile
done
clear
View Answer play_arrow
question_answer 112) Adventitious buds at the leaf notches help to propagate the plant of
A)
potato
done
clear
B)
Agave
done
clear
C)
Bryophyllum
done
clear
D)
Cactus
done
clear
View Answer play_arrow
question_answer 113) Gametogenesis refers to the process of
A)
fusion of two gametes
done
clear
B)
fusion of two gametangia
done
clear
C)
formation of two types of gametes
done
clear
D)
formation of male gamete only
done
clear
View Answer play_arrow
question_answer 114) Stilt roots are found in
A)
Rhizospora
done
clear
B)
maize
done
clear
C)
banyan
done
clear
D)
Colocasia
done
clear
View Answer play_arrow
question_answer 115) In a monocot leaf stomata are present
A)
only on abaxial surface
done
clear
B)
only on adaxial surface
done
clear
C)
on both the surfaces
done
clear
D)
more on abaxial surface
done
clear
View Answer play_arrow
question_answer 116) The inflorescence in which sessile flowers are borne acropetally on an elongated rachis, it is called
A)
raceme
done
clear
B)
spike
done
clear
C)
catkin
done
clear
D)
corymb
done
clear
View Answer play_arrow
question_answer 117) Ring-like secondary cell wall thickening is referred to as
A)
spiral
done
clear
B)
helical
done
clear
C)
scalariform
done
clear
D)
annular
done
clear
View Answer play_arrow
question_answer 118) Aspergillus niger is used for commercial and industrial production of
A)
acetic acid
done
clear
B)
butyric acid
done
clear
C)
citric acid
done
clear
D)
lactic acid
done
clear
View Answer play_arrow
question_answer 119) Statin, a blood-cholesterol lowering agent, is commercially obtained from
A)
Trichoderma polysporum
done
clear
B)
Acetobacter aceti
done
clear
C)
Clostridium butyricum
done
clear
D)
Monascus purpureus
done
clear
View Answer play_arrow
question_answer 120) Adenylic acid is a / an
A)
nitrogen base
done
clear
B)
nucleoside
done
clear
C)
nucleotide
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 121) That plants could be grown to maturity in a defined nutrient solution was for the first time demonstrated by
A)
Priestley
done
clear
B)
Van Sacchs
done
clear
C)
Ingenhausz
done
clear
D)
Van Niel
done
clear
View Answer play_arrow
question_answer 122) In purple and green bacteria, oxygen is not evolved during photosynthesis because hydrogen donor is
A)
\[{{H}_{2}}O\]
done
clear
B)
\[{{H}_{2}}S\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 123) In PS-I, the reaction centre chlorophyll a has an absorption peak at
A)
650 nm
done
clear
B)
660 nm
done
clear
C)
680 nm
done
clear
D)
700 nm
done
clear
View Answer play_arrow
question_answer 124) The idea of two pigment systems in light reaction of photosynthesis was given by
A)
Arnon
done
clear
B)
Hill
done
clear
C)
Blackman
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 125) In respiration, more than one R.Q. value results from the use of
A)
glucose
done
clear
B)
sucrose
done
clear
C)
fat
done
clear
D)
organic acid
done
clear
View Answer play_arrow
question_answer 126) In lactic acid fermentation, the number of ATP formed by the oxidation of NADH is
A)
eight
done
clear
B)
six
done
clear
C)
three
done
clear
D)
Nil
done
clear
View Answer play_arrow
question_answer 127) During aerobic respiration, acetyl Co-A is synthesized in
A)
cytosol
done
clear
B)
mitochondrial matrix.
done
clear
C)
glyoxysomal matrix
done
clear
D)
perichondrial space
done
clear
View Answer play_arrow
question_answer 128) The cell attaining their maximal size in terms of wall thickening and protoplasmic modification belong to
A)
meristematic phase of growth
done
clear
B)
elongation phase of growth
done
clear
C)
maturation phase of growth
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 129) Spraying sugarcane crop with a plant hormone increases the length of the stem, thus increasing the yield by as much as 20 tonnes per acre. The plant hormone is
A)
gibberellin ensymes
done
clear
B)
auxin
done
clear
C)
cytokinin
done
clear
D)
abscisic acid
done
clear
View Answer play_arrow
question_answer 130) The principal function of Golgi apparatus is
A)
producing enzymes
done
clear
B)
protein synthesis
done
clear
C)
synthesis of lipids
done
clear
D)
packaging materials
done
clear
View Answer play_arrow
question_answer 131) A scorpioid cyme having all the flowers in the same plane is
A)
rhipidium
done
clear
B)
bostryx
done
clear
C)
cincinnus
done
clear
D)
dreponium
done
clear
View Answer play_arrow
question_answer 132) Colletrotrichum is an example of
A)
Basidiomycetes
done
clear
B)
Deuteromycetes
done
clear
C)
Ascomycetes
done
clear
D)
Phycomycetes
done
clear
View Answer play_arrow
question_answer 133) A nitrogenous base is linked to the pentose sugar through
A)
hydrogen bond
done
clear
B)
glycosidic bond
done
clear
C)
phosphate diester bond
done
clear
D)
peptide bond
done
clear
View Answer play_arrow
question_answer 134) A mechanism that can cause a gene to move from one linkage group to another is
A)
translocation
done
clear
B)
inversion
done
clear
C)
crossing-over
done
clear
D)
duplication
done
clear
View Answer play_arrow
question_answer 135) Cell cycle includes the sequence
A)
\[S,{{G}_{1}},{{G}_{2}},M\]
done
clear
B)
\[S,M,{{G}_{1}},{{G}_{2}}\]
done
clear
C)
\[{{G}_{1}},S,{{G}_{2}},M\]
done
clear
D)
\[M,{{G}_{1}},{{G}_{2}},S\]
done
clear
View Answer play_arrow
question_answer 136) When the concentration of the solutes is low in plant cell, absorption of water is
A)
retarded
done
clear
B)
increased
done
clear
C)
normal
done
clear
D)
absent
done
clear
View Answer play_arrow
question_answer 137) A citizen group called Friends of the Arcata Marsh (FOAM) basically belongs to
A)
Germany
done
clear
B)
USA
done
clear
C)
Canada
done
clear
D)
UK
done
clear
View Answer play_arrow
question_answer 138) Which of the following is an example of alien species invading a new ecosystem resulting in biodiversity losses?
A)
Introduction of Nile Perch into Lake Victoria in East Africa
done
clear
B)
Introduction of Water Hyacinth into India
done
clear
C)
Introduction of African Catfish into Indian rivers
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 139) The prime contaminants in lakes eutrophied by sewage and agricultural wastes are
A)
sulphates and phosphates
done
clear
B)
nitrates and sulphates
done
clear
C)
nitrates and phosphates
done
clear
D)
nitrates and carbonates
done
clear
View Answer play_arrow
question_answer 140) The process of nutrient enrichment of water, and subsequent loss of species diversity is referred to as
A)
bio concentration
done
clear
B)
bio magnification
done
clear
C)
eutrophication
done
clear
D)
nitrification
done
clear
View Answer play_arrow
question_answer 141) In which cell organelles the genome system is autonomous?
A)
Ribosomes and chloroplasts
done
clear
B)
Mitochondria and chloroplasts
done
clear
C)
Mitochondria and ribosomes
done
clear
D)
Golgi bodies and ribosomes
done
clear
View Answer play_arrow
question_answer 142) Who gave the concept that for a double stranded DNA the ratio between A = T and G = C are equal and constant?
A)
Watson and Crick
done
clear
B)
Wilkins and Franklin
done
clear
C)
Wilkins and Chargaff
done
clear
D)
Chargaff
done
clear
View Answer play_arrow
question_answer 143) In a dihybrid cross between two heterozygotes AaBb \[\times \] AaBb, if we get 3 : 1 ratio among the off springs, the reason for this may be
A)
polygenes
done
clear
B)
linked genes
done
clear
C)
pleiotropic genes
done
clear
D)
hypostatic genes
done
clear
View Answer play_arrow
question_answer 144) What result Mendel would have got, had he self-pollinated a tall \[{{F}_{2}}\] -plant?
A)
TT and Tt
done
clear
B)
All Tf
done
clear
C)
AII TT
done
clear
D)
All ff
done
clear
View Answer play_arrow
question_answer 145) Which of the antibiotic is not produced by one of the Monera (Streptomyces)?
A)
Erythromycin
done
clear
B)
Penicillin
done
clear
C)
Streptomycin
done
clear
D)
Tetramydn
done
clear
View Answer play_arrow
question_answer 146) Entomophilous flowers are
A)
brightly coloured and produce nector
done
clear
B)
colourless
done
clear
C)
inconspicuous
done
clear
D)
odourless
done
clear
View Answer play_arrow
question_answer 147) Which structural level enables the proteins to function as enzymes?
A)
Primary
done
clear
B)
Secondary
done
clear
C)
Tertiary
done
clear
D)
Quaternary
done
clear
View Answer play_arrow
question_answer 148) A distinguishing feature of latex cells is that they are
A)
single-celled elements with anastomosing (fusing) branches
done
clear
B)
single-celled elements with non anastomosingi branches
done
clear
C)
multi-celled elements with anastomosingi branches
done
clear
D)
multi-celled elements with non anastomosing branches
done
clear
View Answer play_arrow
question_answer 149) An organic non-protein cofactor which is easily separable from the apoenzyme is called
A)
prosthetic group
done
clear
B)
coenzyme
done
clear
C)
ailoenzyme
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 150) An immature male gametophyte differs from a mature male gametophyte in that it
A)
has not yet left the pollen sac
done
clear
B)
has not yet germinated and its generative cell has not divided into two male gamete
done
clear
C)
is a microspore that has not yet divided by mitosis
done
clear
D)
still consist of microsporocyte
done
clear
View Answer play_arrow
question_answer 151) A stretch of DNA consisting of 10-20 bases is| most appropriately be called as
A)
polynucleotide
done
clear
B)
nucleotides
done
clear
C)
nucleosides
done
clear
D)
oligonucleotide
done
clear
View Answer play_arrow
question_answer 152) Which biomolecules other than proteins can be catabolised by humans or apes for the production of uric acid?
A)
Carbohydrates
done
clear
B)
Lipids
done
clear
C)
Nucleic acids
done
clear
D)
Vitamins
done
clear
View Answer play_arrow
question_answer 153) The 51 amino acids of insulin are arranged in
A)
single polypeptide
done
clear
B)
two polypeptide having 20 and 31 amino acids
done
clear
C)
two polypeptide having 25 and 26 amino acids
done
clear
D)
two polypeptide having 18 and 33 amino acids
done
clear
View Answer play_arrow
question_answer 154) Substrate of an enzyme is
A)
the reactant of the reaction catalysed by the enzyme
done
clear
B)
competitive inhibitor of the enzyme
done
clear
C)
uncompetitive inhibitor of the enzyme
done
clear
D)
prosthetic group of the enzyme
done
clear
View Answer play_arrow
question_answer 155) Which of the following statements regarding enzymes is not true?
A)
Enzymes lower the activation energy of a reaction
done
clear
B)
Enzymes speed up the attainment of equilibrium of the reaction
done
clear
C)
Enzyme change the value of equilibrium constant of the reaction
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 156) Mitochondrial porins are located in
A)
the outer membrane
done
clear
B)
the inner membrane
done
clear
C)
inter-membrane space
done
clear
D)
Both outer and inner membranes
done
clear
View Answer play_arrow
question_answer 157) Of the total number of genes estimated in human genome, nearly 10 percent are contained in
A)
chromosome 11
done
clear
B)
chromosome 21
done
clear
C)
Y-chromosome
done
clear
D)
chromosome 1
done
clear
View Answer play_arrow
question_answer 158) Human haemoglobin contains
A)
secondary structure
done
clear
B)
tertiary structure
done
clear
C)
quaternary structure
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 159) Which of the following is correct?
A)
Population change = (Birth + immigration) - (death + emigration)
done
clear
B)
Population change = (Birth + immigration) + (death + emigration)
done
clear
C)
Population change = (Birth + emigration) + (death - inmigration)
done
clear
D)
Population change = (Birth - immigration) - (death + emigration)
done
clear
View Answer play_arrow
question_answer 160) In order to lessen the suffering of phenylketonurics their diet should have
A)
no phenylalanine and no tyrosine
done
clear
B)
low phenylalanine and normal requirement of tyrosine
done
clear
C)
normal recommended amount of phenylalanine
done
clear
D)
normal recommended amount of both phenylalanine and tyrosine
done
clear
View Answer play_arrow
question_answer 161) The technique of DNA fingerprinting relies on
A)
repetitive DNA
done
clear
B)
mini-satellite DNA
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 162) During polynucleotide synthesis by the following enzymes, in which case elongation occurs in \[5\to 3\] direction?
A)
DNA polymerase
done
clear
B)
RNA polymerase
done
clear
C)
Reverse transcriptase
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 163) Which of the following types of RNA act as adapter molecule
A)
RNA
done
clear
B)
rRNA
done
clear
C)
mRNA
done
clear
D)
pre-mRNA
done
clear
View Answer play_arrow
question_answer 164) In DNA fingerprinting, the process of DNA hybridisation with the help of specific DNA probe is described as
A)
Western blotting
done
clear
B)
Northern blotting
done
clear
C)
Southern blotting
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 165) In order to induce the bacterial uptake of plasmids, the bacteria are made competent by first treating with
A)
sodium chloride
done
clear
B)
potassium chloride
done
clear
C)
magnesium chloride
done
clear
D)
calcium chloride
done
clear
View Answer play_arrow
question_answer 166) HIV, responsible for AIDS in human is a
A)
ss DNA virus
done
clear
B)
ds DNA virus
done
clear
C)
ss RNA virus
done
clear
D)
ds RNA virus
done
clear
View Answer play_arrow
question_answer 167) Heterogeneous nuclear RNA (hnRNA) is converted to wRNA by
A)
splicing
done
clear
B)
capping
done
clear
C)
tailing .
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 168) The immunoglobulin disulfides do not join
A)
two heavy chains
done
clear
B)
light chains with heavy chains
done
clear
C)
two light chains
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 169) Which of the following techniques is most widely employed to check the progress of a restriction enzyme digestion
A)
Agarose gel electrophoresis
done
clear
B)
Centrifugation
done
clear
C)
Polyacrylamide gel electrophoresis
done
clear
D)
PCR
done
clear
View Answer play_arrow
question_answer 170) The sticky ends on each of the two strands of a DNA generated by treatment with restriction enzymes facilitate the action of enzyme
A)
DNA ligase
done
clear
B)
endonuclease
done
clear
C)
exonuclease
done
clear
D)
DNA polymerase
done
clear
View Answer play_arrow
question_answer 171) Protein encoded by gene cry I Ab controls the infestation of which of the following insects
A)
cotton bollworm
done
clear
B)
Anopheles mosquito
done
clear
C)
corn borer
done
clear
D)
Aedes mosquito
done
clear
View Answer play_arrow
question_answer 172) The first antibody to appear in the serum following stimulation by antigen is
A)
IgM
done
clear
B)
IgG
done
clear
C)
IgA
done
clear
D)
IgE
done
clear
View Answer play_arrow
question_answer 173) Which of the following components are involved in allergic reaction
A)
IgE and mast cells
done
clear
B)
IgG and mast cells
done
clear
C)
IgA and mast cells
done
clear
D)
IgG and basophiles
done
clear
View Answer play_arrow
question_answer 174) The location where B-lymphocytes differentiate and mature is in the
A)
bone marrow
done
clear
B)
bursa of fabric us
done
clear
C)
Both (a) and (b)
done
clear
D)
thymus
done
clear
View Answer play_arrow
question_answer 175) My asthenia gravis is an example of
A)
viral diseases
done
clear
B)
immune deficient diseases
done
clear
C)
auto-immune diseases
done
clear
D)
allergic reactions
done
clear
View Answer play_arrow
question_answer 176) Binomial nomenclature means
A)
one name given by two taxonomists
done
clear
B)
two names, the Latinized, other of a person
done
clear
C)
two names, one scientific, other local
done
clear
D)
two word names, the first indicates genus, and other species
done
clear
View Answer play_arrow
question_answer 177) Which of the following are not eukaryotes?
A)
Monera
done
clear
B)
Protista
done
clear
C)
Animals
done
clear
D)
Plants
done
clear
View Answer play_arrow
question_answer 178) Name of a disease caused by a bacterium
A)
plague
done
clear
B)
mumps
done
clear
C)
dengue
done
clear
D)
sleeping sickness
done
clear
View Answer play_arrow
question_answer 179) DPT vaccine, a combination vaccine, is effective in humans against
A)
Diabetes, Polio and Tetanus
done
clear
B)
Diphtheria, Plague and Tetanus
done
clear
C)
Diphtheria, Pertussis and Typhoid
done
clear
D)
Diphtheria, Pertussis and Tetanus
done
clear
View Answer play_arrow
question_answer 180) The pathogen Haemophilus influenzae is responsible for the disease
A)
influenza
done
clear
B)
pneumonia
done
clear
C)
plague
done
clear
D)
diphtheria
done
clear
View Answer play_arrow
question_answer 181) Ringworm, a common infectious disease in man causing dry scaly lesions on the skin and scalp, is caused by
A)
bacteria
done
clear
B)
roundworms
done
clear
C)
filarial worms
done
clear
D)
fungi
done
clear
View Answer play_arrow
question_answer 182) The sound producing organ of bird is called as
A)
syrinx
done
clear
B)
larynx
done
clear
C)
trachea
done
clear
D)
glottis
done
clear
View Answer play_arrow
question_answer 183) According to IUCN Red list, during the last two decades, the maximum increase in the number of threatened species is among
A)
amphibians
done
clear
B)
reptiles
done
clear
C)
birds
done
clear
D)
mammals
done
clear
View Answer play_arrow
question_answer 184) Appropriate term for the grafting of tissues/organs between the individuals of different species is known as
A)
allograft
done
clear
B)
isograft
done
clear
C)
xenograft
done
clear
D)
transgraft
done
clear
View Answer play_arrow
question_answer 185) Sexual reproduction does not occur in
A)
Ascomycetes
done
clear
B)
Deuteromycetes
done
clear
C)
Basidiomycetes
done
clear
D)
Phycomycetes
done
clear
View Answer play_arrow
question_answer 186) A type of metamorphosis in insects with four developmental stages, i.e., eggs, larva, pupa and adult is
A)
hemimetabolous
done
clear
B)
paurometabolous
done
clear
C)
holometabolous
done
clear
D)
ametabolous
done
clear
View Answer play_arrow
question_answer 187) Which of the following genetic code has a dual functions-coding Met and acts as initiator codon
A)
UAA
done
clear
B)
AUG
done
clear
C)
UUU
done
clear
D)
UAG
done
clear
View Answer play_arrow
question_answer 188) The process of conversion of nitrogen to ammonia by the microbes is described as
A)
nitrification
done
clear
B)
denitrification
done
clear
C)
nitrogen-fixation
done
clear
D)
Habers process
done
clear
View Answer play_arrow
question_answer 189) The type of transport taking place across the bio-membranes without the help of proteins is
A)
facilitated diffusion
done
clear
B)
active transport
done
clear
C)
simple diffusion
done
clear
D)
diffusion via symport
done
clear
View Answer play_arrow
question_answer 190) 70 S ribosomes occur in
A)
bacteria
done
clear
B)
mitochondria
done
clear
C)
chloroplasts
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 191) Which of the following are not ureotelic?
A)
Mammals
done
clear
B)
Terrestrial amphibians
done
clear
C)
Aquatic insects
done
clear
D)
Birds
done
clear
View Answer play_arrow
question_answer 192) The hissardale is a breed of sheep developed by crossing between
A)
Bikaneri ewes and Marino rams
done
clear
B)
Marino ewes and Bikaneri rams
done
clear
C)
Deccani ewes and Bikaneri rams
done
clear
D)
Marino ewes and Apennine rams
done
clear
View Answer play_arrow
question_answer 193) In habitual alcohol drinkers liver gets damaged because of
A)
accumulation of excess glycogen
done
clear
B)
accumulation of excess fat
done
clear
C)
excess detoxification
done
clear
D)
excess deamination
done
clear
View Answer play_arrow
question_answer 194) Which is the hormonal method of birth control?
A)
Pill
done
clear
B)
IUD
done
clear
C)
Vasectomy
done
clear
D)
Femidom
done
clear
View Answer play_arrow
question_answer 195) Sex determination by chromosomal difference in man and Drosophila is by mechanism called
A)
XX-XY
done
clear
B)
XX-XO
done
clear
C)
ZZ-ZW
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 196) Which of the following set of syndromes show 47 chromosomes in their genetic make up
A)
Downs syndrome, Pataus syndrome, Edwards syndrome
done
clear
B)
Turners syndrome, Edwards syndrome, Klinefelters syndrome
done
clear
C)
Klinefelters syndrome, Turners syndrome, Edwards syndrome
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 197) The following is/are removed during hemodialysis
A)
urea
done
clear
B)
glucose
done
clear
C)
amino acid
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 198) Genetic disorder hemophilia is characterized by excessive loss of blood. Which of the following statement is not true in relation to this disease?
A)
It is an autosomal disease
done
clear
B)
It is a X-linked disease
done
clear
C)
Any of the factor VIII or IX may be absent
done
clear
D)
It is a lethal disease
done
clear
View Answer play_arrow
question_answer 199)
Observe the two structural formulae shown below are
A)
isomers
done
clear
B)
epimers
done
clear
C)
anomers
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 200) Fish proteins are considered nutritionally superior to most vegetable proteins because they are rich in
A)
all the 20 amino acids
done
clear
B)
essential amino acids
done
clear
C)
peptide bonds
done
clear
D)
polypeptides
done
clear
View Answer play_arrow