A) its dissociation into\[{{H}^{+}}\]and\[C{{H}_{3}}CO{{O}^{-}}\] ions
B) colligative property measurement
C) molecular association through H-bonding
D) none of the above
Correct Answer: C
Solution :
: \[2C{{H}_{3}}-COOH\]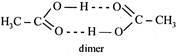
You need to login to perform this action.
You will be redirected in
3 sec
