A) \[N{{i}^{2+}}\]
B) \[S{{c}^{3+}}\]
C) \[C{{u}^{+}}\]
D) \[Z{{n}^{2+}}\]
Correct Answer: A
Solution :
Key Idea: The ions having unpaired electron are paramagnetic. The ions having Paired electrons are diamagnetic. (a)\[N{{i}^{2+}}=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{0}},3{{d}^{8}}\]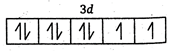 \[\therefore \]It has 2 unpaired electrons and is paramagnetic. (b) \[S{{c}^{3+}}=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{0}},3{{d}^{0}}\] \[\therefore \]It has no unpaired electrons and is diamagnetic (c) \[C{{u}^{+}}=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{0}},3{{d}^{10}}\] \[\therefore \]It has no unpaired electrons and is diamagnetic. (d) \[Z{{n}^{2+}}=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{0}},3{{d}^{10}}\] \[\therefore \] It has no unpaired electrons and is diamagnetic.
\[\therefore \]It has 2 unpaired electrons and is paramagnetic. (b) \[S{{c}^{3+}}=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{0}},3{{d}^{0}}\] \[\therefore \]It has no unpaired electrons and is diamagnetic (c) \[C{{u}^{+}}=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{0}},3{{d}^{10}}\] \[\therefore \]It has no unpaired electrons and is diamagnetic. (d) \[Z{{n}^{2+}}=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},4{{s}^{0}},3{{d}^{10}}\] \[\therefore \] It has no unpaired electrons and is diamagnetic.
You need to login to perform this action.
You will be redirected in
3 sec
