A) \[{{C}_{2}}{{H}_{2}}\]
B) \[N{{H}_{3}}\]
C) \[{{N}_{2}}O\]
D) None of these
Correct Answer: B
Solution :
\[\text{N}{{\text{H}}_{\text{3}}}\]is not planar molecule because it has \[\text{s}{{\text{p}}^{\text{3}}}\] hybridisation and pyramidal shape. C in\[{{C}_{2}}{{H}_{2}}\] is sp hybridized so it is planar. \[N\equiv \overset{\oplus }{\mathop{N}}\,-{{O}^{o-}}\] (a) \[H-C\equiv C-H\] (b)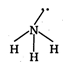
You need to login to perform this action.
You will be redirected in
3 sec
