A) 7 cm
B) 5 cm
C) 4.5 cm
D) 2.3 cm
Correct Answer: B
Solution :
Key Idea: An isothermal process obeys Boyles law. Since, process is isothermal the total a pressure of air inside bubble is same as excess of pressure given by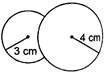 where T is surface tension and R is radius. Also, an isothermal process obeys Boyles law. Hence, PV= constant. Let R be the radius of coalesce system, then \[{{P}_{1}}{{V}_{1}}+{{P}_{2}}{{V}_{2}}=PV\] \[\left( \frac{4T}{{{R}_{1}}} \right)\left( \frac{4}{3}\pi R_{1}^{3} \right)+\left( \frac{4T}{{{R}_{2}}} \right)\left( \frac{4}{3}\pi R_{2}^{3} \right)=\left( \frac{4T}{R} \right)\left( \frac{4}{3}\pi {{R}^{3}} \right)\] \[\Rightarrow \] \[r_{1}^{2}+r_{2}^{2}={{R}^{2}}\] Given, \[{{R}_{1}}=3\,\,cm,\,\,{{R}_{2}}=4\,\,cm\] \[\therefore \] \[R=\sqrt{{{3}^{2}}+{{4}^{2}}}\] \[\Rightarrow \] \[R=5\,\,cm\]
where T is surface tension and R is radius. Also, an isothermal process obeys Boyles law. Hence, PV= constant. Let R be the radius of coalesce system, then \[{{P}_{1}}{{V}_{1}}+{{P}_{2}}{{V}_{2}}=PV\] \[\left( \frac{4T}{{{R}_{1}}} \right)\left( \frac{4}{3}\pi R_{1}^{3} \right)+\left( \frac{4T}{{{R}_{2}}} \right)\left( \frac{4}{3}\pi R_{2}^{3} \right)=\left( \frac{4T}{R} \right)\left( \frac{4}{3}\pi {{R}^{3}} \right)\] \[\Rightarrow \] \[r_{1}^{2}+r_{2}^{2}={{R}^{2}}\] Given, \[{{R}_{1}}=3\,\,cm,\,\,{{R}_{2}}=4\,\,cm\] \[\therefore \] \[R=\sqrt{{{3}^{2}}+{{4}^{2}}}\] \[\Rightarrow \] \[R=5\,\,cm\]
You need to login to perform this action.
You will be redirected in
3 sec
