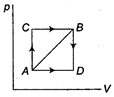
| I. Increase in internal energy from state A to state B is 50 J. |
| II. If path ADB is followed to reach state B,\[\Delta E=50\,J\]. |
| III. If work done by the system in path AB is 20 J, the heat absorbed during path AB = 70 J. |
| IV. The value \[{{E}_{C}}-{{E}_{A}}\] is equal to \[{{E}_{D}}-{{E}_{B}}\]. |
| V. Heat absorbed by the system to reach B from A through path ADB is 60 J. |
A) l, V
B) l, III, V
C) I, II. Ill, V
D) l, IV, V
Correct Answer: C
Solution :
\[ACB=AC+BC\] Heat absorbed 80 J. Work done by the system = 10 J \[\therefore \] \[W=-10\,J\] \[\therefore \] \[W=-30\]Also \[W=-10\,J\] \[\therefore \] \[{{E}_{B}}-{{E}_{A}}=50\,J\] \[\therefore \] \[Q=50+10=60\,J\]You need to login to perform this action.
You will be redirected in
3 sec
