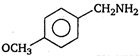
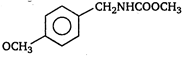
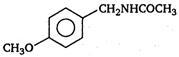
question_answer 1) A body is vibrating in simple harmonic motion with an amplitude of 0.06 m and frequency of 15 Hz. The maximum velocity and acceleration of the body is:
A)
9.80 m/s and \[9.03\times {{10}^{2}}m/{{s}^{2}}\]
done
clear
B)
8.90 m/s and \[8.21\times {{10}^{2}}m/{{s}^{2}}\]
done
clear
C)
6.82 m/s and \[7.62\times {{10}^{2}}m/{{s}^{2}}\]
done
clear
D)
5.65 m/s and \[5.32\times {{10}^{2}}m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 2) A motor cycle is travelling on a curved track of radius 500 m. If the coefficient of friction between tyres and road is 0.5 with\[g=10\,\,m/{{s}^{2}}\], what should be the maximum speed to avoid skidding?
A)
10 m/s
done
clear
B)
50 m/s
done
clear
C)
250 m/s
done
clear
D)
500 m/s
done
clear
View Answer play_arrow
question_answer 3) If the equation of motion of standing wave is \[y=0.3\sin \,(314\,t-1.57\,x)\],the velocity of standing wave is :
A)
400 unit
done
clear
B)
250 unit
done
clear
C)
200 unit
done
clear
D)
150 unit
done
clear
View Answer play_arrow
question_answer 4) On producing the waves of frequency 1000 Hz in a Kundts tube, the total distance between 6 successive nodes is 85 cm. Speed of sound in the gas filled in the tube is :
A)
300 m/s
done
clear
B)
350 m/s
done
clear
C)
340 m/s
done
clear
D)
330 m/s
done
clear
View Answer play_arrow
question_answer 5) A spring is vibrating with frequency under same mass. If it is cut into two equal pieces and same mass is suspended, then the new frequency will be :
A)
\[n\sqrt{2}\]
done
clear
B)
\[\frac{n}{\sqrt{2}}\]
done
clear
C)
\[\frac{n}{2}\]
done
clear
D)
n
done
clear
View Answer play_arrow
question_answer 6) Diamagnetic substances are :
A)
strongly repelled by magnets
done
clear
B)
feebly repelled by magnets
done
clear
C)
feebly attracted by magnets
done
clear
D)
strongly attracted by magnets
done
clear
View Answer play_arrow
question_answer 7) The study of the effects associated with electric field at rest is known as :
A)
electrostatics
done
clear
B)
electromagnetism
done
clear
C)
magnetostatics
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 8) For driving current of 2 A for 6 min in a circuit, 1000 J of work is to be done. The emf of the source in the circuit is :
A)
1.38V
done
clear
B)
1.68V
done
clear
C)
2.03V
done
clear
D)
3.10V
done
clear
View Answer play_arrow
question_answer 9) A current carrying wire in the neighbourhood produces :
A)
electric and magnetic fields
done
clear
B)
magnetic field only
done
clear
C)
no field
done
clear
D)
electric field
done
clear
View Answer play_arrow
question_answer 10) A resonance air column of length 20cm resonates with a tuning fork of frequency 250 Hz. The speed of the air is:
A)
75 m/s
done
clear
B)
150 m/s
done
clear
C)
200 m/s
done
clear
D)
300 m/s
done
clear
View Answer play_arrow
question_answer 11) An iron rod of length 2 m and cross-sectional area of 50 \[m{{m}^{2}}\]is stretched by 0.5 mm, when a mass of 250 kg is hung from its lower end. Youngs modulus of iron rod is :
A)
\[19.6\times {{10}^{20}}N/{{m}^{2}}\]
done
clear
B)
\[19.6\times {{10}^{18}}N/{{m}^{2}}\]
done
clear
C)
\[19.6\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
D)
\[19.6\times {{10}^{15}}N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 12) Frequency of infrared wave is approximately:
A)
1018 Hz
done
clear
B)
1014 Hz
done
clear
C)
109 Hz
done
clear
D)
1016 Hz
done
clear
View Answer play_arrow
question_answer 13) A wire has resistance of \[3.1\,\,\Omega \] at \[{{30}^{o}}C\] and resistance \[4.5\,\,\Omega \] at \[{{100}^{o}}C\]. The temperature coefficient of resistance of the wire is :
A)
\[{{0.0012}^{o}}{{C}^{-1}}\]
done
clear
B)
\[{{0.0024}^{o}}{{C}^{-1}}\]
done
clear
C)
\[{{0.0032}^{o}}{{C}^{-1}}\]
done
clear
D)
\[{{0.0064}^{o}}{{C}^{-1}}\]
done
clear
View Answer play_arrow
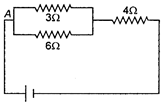
question_answer 14)
If the current through \[3\,\,\Omega \] resistor is 0.8 A, then potential drop through \[4\,\,\Omega \] resistor is :
A)
1.2V
done
clear
B)
2.6V
done
clear
C)
4.8 V
done
clear
D)
9.6 V
done
clear
View Answer play_arrow
question_answer 15) The direction of the null points on the equatorial line of a bar magnet, when the north pole of the magnet is pointing to :
A)
west
done
clear
B)
east
done
clear
C)
south
done
clear
D)
north
done
clear
View Answer play_arrow
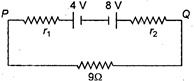
question_answer 16)
Two batteries of emf 4 V and 8 V having the internal resistance of \[1\,\,\Omega \] and \[2\,\,\Omega \]. respectively are connected in circuit with a resistance of \[9\,\,\Omega \] as shown in a figure. The current and potential difference between the points P and Q are :
A)
\[\frac{1}{2}\]A and 12 V
done
clear
B)
\[\frac{1}{9}\]A and 9 V
done
clear
C)
\[\frac{1}{6}\]A and 4 V
done
clear
D)
\[\frac{1}{3}\] A and 3 V
done
clear
View Answer play_arrow
question_answer 17) The dimensions of gravitational constant G are:
A)
\[[M{{L}^{3}}{{T}^{-2}}]\]
done
clear
B)
\[[{{M}^{-1}}{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{-2}}{{T}^{2}}]\]
done
clear
D)
\[[{{M}^{-1}}{{L}^{3}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 18) A 30 g bullet initially travelling at 120 m/s penetrates 12 cm into wooden block. The average resistance exerted by the wooden block is :
A)
1800 N
done
clear
B)
2000 N
done
clear
C)
2200 N
done
clear
D)
2850 N
done
clear
View Answer play_arrow
question_answer 19) Angle of dip is \[{{90}^{o}}\] at:
A)
equator
done
clear
B)
middle point
done
clear
C)
poles
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 20) Two magnets each of magnetic moment M are placed so as to form a cross at right angles to each other. The magnetic moment of the system will be :
A)
M
done
clear
B)
0.5 M
done
clear
C)
V2M
done
clear
D)
2M
done
clear
View Answer play_arrow
question_answer 21) Plate current will be maximum when :
A)
both the grid and anode are negative
done
clear
B)
both the grid and anode are positive
done
clear
C)
grid is positive and anode is negative
done
clear
D)
grid is negative and anode is positive
done
clear
View Answer play_arrow
question_answer 22) Sodium has body centered packing. Distance between two nearest atoms is 3.7 \[\overset{o}{\mathop{A}}\,\]. The lattice parameter is :
A)
4.9 \[\overset{o}{\mathop{A}}\,\]
done
clear
B)
4.3 \[\overset{o}{\mathop{A}}\,\]
done
clear
C)
3.8 \[\overset{o}{\mathop{A}}\,\]
done
clear
D)
3.4 \[\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 23) The instrument used to measure the temperature of the source from its thermal radiation is :
A)
hydrometer
done
clear
B)
barometer
done
clear
C)
thermopile
done
clear
D)
pyrometer
done
clear
View Answer play_arrow
question_answer 24) Which of the following are not the transverse waves?
A)
Sound waves
done
clear
B)
Visible light waves
done
clear
C)
X-rays
done
clear
D)
y-rays
done
clear
View Answer play_arrow
question_answer 25) The displacement x of a particle moving along a straight line at time t is given by \[x={{a}_{0}}+{{a}_{1}}\,t+{{a}_{2}}{{t}^{2}}\] The acceleration of the particle is :
A)
\[4\,{{a}_{2}}\]
done
clear
B)
\[2\,{{a}_{2}}\]
done
clear
C)
\[2\,{{a}_{1}}\]
done
clear
D)
\[{{a}_{2}}\]
done
clear
View Answer play_arrow
question_answer 26) A bomb is dropped from an aeroplane moving horizontally at constant speed. If air resistance is taken into consideration, then the bomb :
A)
falls on earth exactly below the aeroplane
done
clear
B)
falls on the earth exactly behind ±e aeroplane
done
clear
C)
falls on the earth ahead of the aeroplane
done
clear
D)
flies with the aeroplane
done
clear
View Answer play_arrow
question_answer 27) In a p -type semiconductor germanium is doped with :
A)
aluminium
done
clear
B)
boron
done
clear
C)
gallium
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 28) Boolean algebra is essentially based on :
A)
numbers
done
clear
B)
symbol
done
clear
C)
logic
done
clear
D)
truth
done
clear
View Answer play_arrow
question_answer 29) When a bus suddenly takes a turn, the passengers are thrown outwards because of:
A)
speed of motion
done
clear
B)
inertia of motion
done
clear
C)
acceleration of motion
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 30) The logic behind NOR gate is that which gives :
A)
high output when both inputs are high
done
clear
B)
low output when both inputs are low
done
clear
C)
high output when both inputs are low
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 31) How can the chromatic aberration be corrected?
A)
By providing different suitable curvature to its two surfaces
done
clear
B)
By combining it with another lens of opposite nature
done
clear
C)
By reducing its aperture
done
clear
D)
By providing proper polishing of its two surfaces
done
clear
View Answer play_arrow
question_answer 32) Which one of the following is not dependent on the intensity of incident photon in a photoelectric experiment?
A)
Work function of the surface
done
clear
B)
Number of photoelectrons
done
clear
C)
Stopping potential
done
clear
D)
Amount of photoelectric current
done
clear
View Answer play_arrow
question_answer 33) If red light and violet light rays are of focal lengths \[{{f}_{R}}\] and \[{{f}_{V}}\], then which one of the following is true ?
A)
\[{{\lambda }_{R}}\le {{\lambda }_{V}}\]
done
clear
B)
\[{{\mu }_{R}}>{{\mu }_{V}}\]
done
clear
C)
\[{{\lambda }_{R}}={{\lambda }_{V}}\]
done
clear
D)
\[{{\mu }_{R}}<\mu {{\,}_{V}}\]
done
clear
View Answer play_arrow
question_answer 34) The large scale destruction, that would be caused due to the use of nuclear weapons known as :
A)
neutron reproduction factor
done
clear
B)
nuclear holocaust
done
clear
C)
thermonuclear reaction
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 35) If in Ramsdens eyepiece, the field lenses have focal lengths \[{{f}_{1}}\] and \[{{f}_{2}}\] respectively and separated by a distance d then :
A)
\[{{f}_{1}}=3{{f}_{2}}\] and \[d={{f}_{1}}+{{f}_{2}}\]
done
clear
B)
\[{{f}_{1}}={{f}_{2}}\] and \[d=\frac{2}{3}{{f}_{1}}\]
done
clear
C)
\[{{f}_{1}}=\frac{2}{3}{{f}_{2}}\] and \[d=\frac{2}{3}{{f}_{1}}\]
done
clear
D)
\[{{f}_{1}}={{f}_{2}}\] and \[d={{f}_{1}}+{{f}_{2}}\]
done
clear
View Answer play_arrow
question_answer 36) Huygens wave theory of light could not explain :
A)
photoelectric effect
done
clear
B)
polarisation
done
clear
C)
diffraction
done
clear
D)
interference
done
clear
View Answer play_arrow
question_answer 37) The rain drops are spherical in shape due to :
A)
residual pressure
done
clear
B)
thrust on drop
done
clear
C)
surface tension
done
clear
D)
viscosity
done
clear
View Answer play_arrow
question_answer 38) The work done in pulling up a block of wood weighing 2 kN for a length of 10 m on a smooth plane inclined at an angle of \[{{15}^{o}}\] with the horizontal is :
A)
9.82 kJ
done
clear
B)
8.91 kJ
done
clear
C)
5.17 kJ
done
clear
D)
4.36 kJ
done
clear
View Answer play_arrow
question_answer 39) The thermions are :
A)
positrons
done
clear
B)
photons
done
clear
C)
electrons
done
clear
D)
protons
done
clear
View Answer play_arrow
question_answer 40) The internal resistance of cell of emf 2 V is\[0.1\,\,\Omega \]. It is connected to a resistance of \[3.9\,\,\Omega \]. The voltage across the cell is :
A)
2.71 V
done
clear
B)
1.95 V
done
clear
C)
1.68 V
done
clear
D)
0.52 V
done
clear
View Answer play_arrow
question_answer 41) The earth of mass 6 xl024 kg revolves around the sun with an angular velocity of \[2\times {{10}^{-7}}\] rad/s. In a circular orbit of radius \[1.5\times {{10}^{8}}\] km, the force exerted by the sun, on the earth is :
A)
\[27\times {{10}^{39}}N\]
done
clear
B)
\[36\times {{10}^{21}}N\]
done
clear
C)
\[18\times {{10}^{25}}N\]
done
clear
D)
\[6\times {{10}^{19}}N\]
done
clear
View Answer play_arrow
question_answer 42) A stone is thrown with an initial speed of 4.9 m/s from a bridge in vertically upward direction. It falls down in water after 2 s. The height of the bridge is :
A)
24.7 m
done
clear
B)
19.8 m
done
clear
C)
9.8m
done
clear
D)
4.9m
done
clear
View Answer play_arrow
question_answer 43) A ball of mass 150 g moving with an acceleration 20 \[m/{{s}^{2}}\] is hit by a force, which acts, on it for 0.1 s. The impulsive force is:
A)
1.2 N-S
done
clear
B)
0.3 N-s
done
clear
C)
0.1 N-s
done
clear
D)
0.5 N-s
done
clear
View Answer play_arrow
question_answer 44) If the heat of 110 J is added to a gaseous system, whose internal energy is 40 J, then the amount of external work done is :
A)
80 J
done
clear
B)
70 J
done
clear
C)
115 J
done
clear
D)
140 J
done
clear
View Answer play_arrow
question_answer 45) The substance in which the magnetic moment of a single atom is not zero, is called as :
A)
femmagnetism
done
clear
B)
paramagnedsm
done
clear
C)
ferromagnetism
done
clear
D)
diamagnetism
done
clear
View Answer play_arrow
question_answer 46) The luminous efficiency of a lamp is 4 lumen/W and its luminous intensity is 30 Cd. The power of lamp is :
A)
60 W
done
clear
B)
78 W
done
clear
C)
94 W
done
clear
D)
136 W
done
clear
View Answer play_arrow
question_answer 47) The transfer ratio \[\beta \] of a transistor is 50. The input resistance of the transistor when used in the common-emitter configuration is \[1\,k\Omega \]. The peak value of the collector AC current for an AC input voltage of 0.01 V, is :
A)
500 \[\mu A\]
done
clear
B)
0.25 \[\mu A\]
done
clear
C)
0.01 \[\mu A\]
done
clear
D)
100 \[\mu A\]
done
clear
View Answer play_arrow
question_answer 48) Two vectors \[\vec{A}\] and \[\vec{B}\] are such that \[\vec{A}+\vec{B}=\vec{C}\]and \[{{A}^{2}}+{{B}^{2}}={{C}^{2}}\]. If \[\theta \] is the angle between \[\vec{A}\] and \[\vec{B}\] then correct statement is :
A)
\[\theta =\pi \]
done
clear
B)
\[\theta =\frac{2\pi }{3}\]
done
clear
C)
\[\theta =0\]
done
clear
D)
\[\theta =\frac{\pi }{2}\]
done
clear
View Answer play_arrow
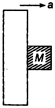
question_answer 49)
A rough vertical board has an acceleration a along the horizontal, so that a block of mass M pressing against it does not fall. The coefficient of friction between block and the board is:
A)
\[>\frac{a}{g}\]
done
clear
B)
\[<\frac{g}{a}\]
done
clear
C)
\[=\frac{a}{g}\]
done
clear
D)
\[>\frac{g}{a}\]
done
clear
View Answer play_arrow
question_answer 50) An aeroplane is moving with a horizontal velocity u at a height h. The velocity of packet dropped from it on the earths surface will be:
A)
\[\sqrt{{{u}^{2}}-2g\,h}\]
done
clear
B)
\[2\,g\,\,h\]
done
clear
C)
\[\sqrt{2\,g\,\,h}\]
done
clear
D)
\[\sqrt{{{u}^{2}}+2\,g\,\,h}\]
done
clear
View Answer play_arrow
question_answer 51) Which of the following having highest number of molecules?
A)
\[16\,g\,{{O}_{2}}\]
done
clear
B)
\[14\,g\,{{N}_{2}}\]
done
clear
C)
\[2\,g\,{{H}_{2}}\]
done
clear
D)
\[6\,g\,{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 52) The size of isoelectronic ions depends on :
A)
ionisation energy
done
clear
B)
nuclear charge
done
clear
C)
ionic radius
done
clear
D)
covalent radius
done
clear
View Answer play_arrow
question_answer 53) At constant temperature the pressure of a gas is increased to three times, then its volume becomes :
A)
\[\frac{V}{3}\]
done
clear
B)
\[\frac{2}{3}V\]
done
clear
C)
3V
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 54) Find the temperature of hydrogen gas which has the same velocity as that of oxygen at a temperature of \[{{0}^{o}}C\]:
A)
\[273\div 16\text{ }K\]
done
clear
B)
\[273\times 8\text{ }K\]
done
clear
C)
\[273\div 32\text{ }K\]
done
clear
D)
\[273\times 4\text{ }K\]
done
clear
View Answer play_arrow
question_answer 55) What is the bond order in case of \[O_{2}^{+}\]?
A)
2.5
done
clear
B)
2
done
clear
C)
1.5
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 56) Lindlars catalyst is :
A)
Pd supported over \[BaC{{O}_{3}}\]
done
clear
B)
Hg supported over \[PbS{{O}_{4}}\]
done
clear
C)
Ni supported over \[CuS{{O}_{4}}\]
done
clear
D)
Ni supported over \[CdS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 57) In \[s{{p}^{3}}\]d-hybridisation, the d-orbital that participate in hybridisation is :
A)
\[dxy\]
done
clear
B)
\[dxz\]
done
clear
C)
\[d{{x}^{2}}-{{y}^{2}}\]
done
clear
D)
\[d{{z}^{2}}\]
done
clear
View Answer play_arrow
question_answer 58) Which of the following is correct in the reaction? \[{{H}_{2}}(g)+{{I}_{2}}(g)\xrightarrow{{}}2HI(g)\]
A)
\[{{K}_{p}}={{K}_{c}}\]
done
clear
B)
\[{{K}_{p}}>{{K}_{c}}\]
done
clear
C)
\[{{K}_{p}}<{{K}_{c}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 59) A \[CaC{{O}_{3}}\] sample contains \[3.01\times {{10}^{23}}\] ions of \[\overset{2+}{\mathop{Ca}}\,\] and \[C{{O}_{3}}^{2-}\]. The mass of sample is:
A)
40
done
clear
B)
50
done
clear
C)
60
done
clear
D)
70
done
clear
View Answer play_arrow
question_answer 60) The increasing order for the value of charge/mass for electron [e], proton [p], neutron [n] and alpha particle [a] is :
A)
\[e<p<n<\alpha \]
done
clear
B)
\[n<p<e<\alpha \]
done
clear
C)
\[n<p<\alpha <e\]
done
clear
D)
\[n<\alpha <p<e\]
done
clear
View Answer play_arrow
question_answer 61) The bond order of \[{{O}_{2}}^{-}\] is :
A)
1
done
clear
B)
zero
done
clear
C)
1.5
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 62) d and \[l\] tartaric acids are:
A)
enantiomers
done
clear
B)
tautomers
done
clear
C)
mesomers
done
clear
D)
diastereomers
done
clear
View Answer play_arrow
question_answer 63) In the reaction, \[2C(s)+{{O}_{2}}(g)2CO\,\,(g)\] the partial pressure of CO and \[{{O}_{2}}\] is 8 atm and 4 arm respectively, then find its equilibrium constant:
A)
16
done
clear
B)
24
done
clear
C)
8
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 64) Which is the correct electronic configuration of of Cr (chromium)?
A)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}3{{d}^{5}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{4}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{6}}\]
done
clear
D)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}3{{d}^{8}}\]
done
clear
View Answer play_arrow
question_answer 65) If heat of neutralisation of \[C{{H}_{3}}COOH\] and \[NaOH\] is -50.6 kJ equivalent and the heat of neutralisation of \[N{{H}_{4}}OH\] and \[HCl\] is -51.4 kJ equivalent then the heat of neutralisation of \[C{{H}_{3}}COOH\] and \[N{{H}_{4}}OH\] is:
A)
102 kJ
done
clear
B)
-0.8 kJ
done
clear
C)
0.8 kJ
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 66) At \[{{25}^{o}}C\,{{K}_{a}}\] for \[C{{H}_{3}}COOH\] is \[1.8\times {{10}^{-5}}\] and \[{{K}_{b}}\] for \[N{{H}_{4}}OH\] is also \[1.8\times {{10}^{-5}}\]. The nature of aqueous solution of \[C{{H}_{3}}COON{{H}_{4}}\] is :
A)
neutral
done
clear
B)
basic
done
clear
C)
acidic
done
clear
D)
amphoteric
done
clear
View Answer play_arrow
question_answer 67) On bombarding \[_{7}{{N}^{14}}\] with \[\alpha \]-particles, the nuclei of the product formed after the release of a proton is :
A)
\[_{8}{{O}^{17}}\]
done
clear
B)
\[_{8}{{O}^{18}}\]
done
clear
C)
\[_{9}{{F}^{7}}\]
done
clear
D)
\[_{9}{{F}^{18}}\]
done
clear
View Answer play_arrow
question_answer 68) Coordination number and oxidation number of Cr in \[{{K}_{3}}Cr{{({{C}_{2}}{{O}_{4}})}_{3}}\] are respectively :
A)
6 and +3
done
clear
B)
4 and-2
done
clear
C)
3 and 0
done
clear
D)
3 and +3
done
clear
View Answer play_arrow
question_answer 69) Thermite is a mixture of:
A)
\[MgO+Al\]
done
clear
B)
\[F{{e}_{3}}{{O}_{4}}+Al\]
done
clear
C)
\[Zn+CaC{{O}_{3}}\]
done
clear
D)
\[Zn+{{P}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 70) Geometrical isomerism is shown by :
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 71) The oxidation state of sulphur in \[{{H}_{2}}S{{O}_{4}}\]:
A)
+6
done
clear
B)
+4
done
clear
C)
+2
done
clear
D)
+3
done
clear
View Answer play_arrow
question_answer 72) For detection of sulphur in an. Organic compound, sodium nitroprusside is added to the Lassaignes filtrate the ppt. obtained is :
A)
purple colour
done
clear
B)
black colour
done
clear
C)
blood-red colour
done
clear
D)
white colour
done
clear
View Answer play_arrow
question_answer 73) Which of the following is correct for\[^{60}Co\xrightarrow{-{{\beta }^{-}}}\]?
A)
\[^{61}Co\]
done
clear
B)
\[^{60}Ni\]
done
clear
C)
\[^{59}Ni\]
done
clear
D)
\[^{60}Cu\]
done
clear
View Answer play_arrow
question_answer 74) Which of the following is not linear?
A)
\[BeC{{l}_{2}}\]
done
clear
B)
\[HCN\]
done
clear
C)
\[ZnC{{l}_{2}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following compound is optically active?
A)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHOHCOOH\]
done
clear
C)
\[HOOC\,.\,C{{H}_{2}}\,.\,COOH\]
done
clear
D)
\[C{{H}_{3}}\,.\,\,CO\,.\,COOH\]
done
clear
View Answer play_arrow
question_answer 76) The boiling point of phenols are higher than the hydrocarbons of comparable masses due to:
A)
more polarising power
done
clear
B)
presence of hydrogen bonding
done
clear
C)
resonance stabilisation
done
clear
D)
acidic character
done
clear
View Answer play_arrow
question_answer 77)
The product obtained is :
A)
benzoic acid
done
clear
B)
benzaldehyde
done
clear
C)
salicylaldehyde
done
clear
D)
salicylic acid
done
clear
View Answer play_arrow
question_answer 78) The reduction of aldehyde and ketones to the corresponding hydrocarbons with amalgamated zinc and concentrated \[HCl\] is called :
A)
Wolff- Kishner reduction
done
clear
B)
Clemmensen reduction
done
clear
C)
Coupling reduction
done
clear
D)
Cross- Cannizaro reaction
done
clear
View Answer play_arrow
question_answer 79) The enthalpy and entropy change for a reaction is \[-2.5\times {{10}^{3}}\,cal\] and 7.4 cal \[{{\deg }^{-1}}\]respectively. At 298 K the reaction is:
A)
reversible
done
clear
B)
irreversible
done
clear
C)
spontaneous
done
clear
D)
non-spontaneous
done
clear
View Answer play_arrow
question_answer 80) The energy of an electron in the first Bohr orbit is -13.6 eV, then find the energy of \[H{{e}^{+}}\] in the same Bohr orbit:
A)
27.2 eV
done
clear
B)
-27.2 eV
done
clear
C)
54.4 eV
done
clear
D)
-54.4 eV
done
clear
View Answer play_arrow
question_answer 81) Aldehydes and ketones can be distinguished by:
A)
Tollens reagent
done
clear
B)
2, 4 DNP test
done
clear
C)
Molisch test
done
clear
D)
Mullikans test
done
clear
View Answer play_arrow
question_answer 82) The electrical conductance is shown by :
A)
sodium
done
clear
B)
diamond
done
clear
C)
graphite
done
clear
D)
potassium
done
clear
View Answer play_arrow
question_answer 83) The largest number of molecules are present in:
A)
\[5g\,N{{H}_{3}}\]
done
clear
B)
\[11\,g\,C{{O}_{2}}\]
done
clear
C)
\[8\,g\,S{{O}_{2}}\]
done
clear
D)
\[4\,g\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 84) The splitting of spectral lines under the influence of magnetic field is known as :
A)
Zeeman effect
done
clear
B)
photoelectric effect
done
clear
C)
Stark effect
done
clear
D)
electromagnetic effect
done
clear
View Answer play_arrow
question_answer 85) Write the product: \[\begin{matrix} C{{H}_{2}}OH \\ \begin{align} & | \\ & C{{H}_{2}}OH \\ \end{align} \\ \end{matrix}\xrightarrow{HI{{O}_{4}}}\]
A)
formaldehyde
done
clear
B)
acetaldehyde
done
clear
C)
formic acid
done
clear
D)
acetic acid
done
clear
View Answer play_arrow
question_answer 86) lonizarion energy of hydrogen is :
A)
slightly higher than chlorine
done
clear
B)
much higher than chlorine
done
clear
C)
lesser than chlorine
done
clear
D)
equal to chlorine
done
clear
View Answer play_arrow
question_answer 87) In LPG gas the main constituent of the gas is :
A)
ethane
done
clear
B)
methane
done
clear
C)
butane
done
clear
D)
propane
done
clear
View Answer play_arrow
question_answer 88) Which plays a major role in the formation of complex compound?
A)
Transition metal
done
clear
B)
Lanthanides and actinides
done
clear
C)
Representative elements
done
clear
D)
p-block element
done
clear
View Answer play_arrow
question_answer 89) The conjugate base of \[{{H}_{2}}PO_{4}^{-}\] is :
A)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
B)
\[HP{{O}_{4}}^{2-}\]
done
clear
C)
\[P{{O}_{4}}^{3-}\]
done
clear
D)
\[{{H}_{2}}P{{O}_{3}}^{-}\]
done
clear
View Answer play_arrow
question_answer 90)
The product obtained from :
A)
done
clear
B)
done
clear
C)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 91) Benzaldehyde on treatment with ethanolic KCN produce :
A)
\[{{C}_{6}}{{H}_{5}}COCO{{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CHOHCN\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}CHOHCOOH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}CHOHCO{{C}_{6}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 92) When aniline is warm with \[CHC{{l}_{3}}\] and ale.\[KOH\], it forms a compound which having offensive smell, the compound formed is :
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 93) The formation of large number of compounds of carbon is due to its :
A)
non-metallic nature
done
clear
B)
catenation character
done
clear
C)
high ionisation potential
done
clear
D)
four valency
done
clear
View Answer play_arrow
question_answer 94) Orthophosphoric acid is :
A)
monobasic
done
clear
B)
dibasic
done
clear
C)
tribasic
done
clear
D)
tetrabasic
done
clear
View Answer play_arrow
question_answer 95) The volume strength of 1,5 (N) \[{{H}_{2}}{{O}_{2}}\] solution is:
A)
4.8
done
clear
B)
8.4
done
clear
C)
3
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 96) A gas has a vapour density 11.2. The volume occupied by 1 g of gas at NTP is :
A)
1 L
done
clear
B)
11.2 L
done
clear
C)
22.4 L
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
question_answer 97) What is the rate of a reaction in a first order reaction, if its half life period is 693 s and its concentration is 2 mol/L?
A)
100
done
clear
B)
0.002
done
clear
C)
0.02
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 98)
Write the product formed in the reaction :
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 99) The most radioactive element is :
A)
radium
done
clear
B)
uranium
done
clear
C)
polonium
done
clear
D)
thorium
done
clear
View Answer play_arrow
question_answer 100) A compound contains 38.8% C, 16% H and 45.2% N. The empirical formula of the compound will be :
A)
\[C{{H}_{5}}N\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}N\]
done
clear
C)
\[CHN\]
done
clear
D)
\[C{{H}_{10}}{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 101) Malignant tertian malaria is caused by :
A)
Plasmodium falciparum
done
clear
B)
P. ovale
done
clear
C)
P. vivax
done
clear
D)
P. malariae
done
clear
View Answer play_arrow
question_answer 102) Agar-agar is extracted from :
A)
Selaginella
done
clear
B)
Spirogyra
done
clear
C)
Gelidium
done
clear
D)
Diatoms
done
clear
View Answer play_arrow
question_answer 103) Cause of death during snake bite :
A)
failure of nerves
done
clear
B)
destruction of RBCs
done
clear
C)
permanent contraction of muscles
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 104) The metal ion involved in stomatal regulation is:
A)
Fe
done
clear
B)
Mg
done
clear
C)
Zn
done
clear
D)
K
done
clear
View Answer play_arrow
question_answer 105) Oviparous mammals are :
A)
kangaroo
done
clear
B)
duck billed platypus
done
clear
C)
whale
done
clear
D)
rabbit
done
clear
View Answer play_arrow
question_answer 106) Oxygen which is liberated during photosynthesis comes from:
A)
\[C{{O}_{2}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
phosphoglyceric acid
done
clear
D)
chlorophyll
done
clear
View Answer play_arrow
question_answer 107) In Singer and Nicolson model of plasma membrane/the extrinsic proteins are :
A)
tightly associated with intrinsic protein and can be easily separated
done
clear
B)
loosely associated with intrinsic protein and can be easily separated
done
clear
C)
loosely associated with intrinsic protein and cant be easily separated
done
clear
D)
tightly associated with intrinsic protein and cant be easily separated
done
clear
View Answer play_arrow
question_answer 108) Ribosomes are associated with:
A)
RNA synthesis
done
clear
B)
protein synthesis
done
clear
C)
enzyme mobilisation
done
clear
D)
DNA synthesis
done
clear
View Answer play_arrow
question_answer 109) Minimata disease caused by water pollution is due to:
A)
lead poisoning
done
clear
B)
arsenic chloride poisoning
done
clear
C)
mercuric poisoning
done
clear
D)
ammonia pollution
done
clear
View Answer play_arrow
question_answer 110) Abscisic acid controls:
A)
cell elongation and cell wall formation
done
clear
B)
shoot elongation
done
clear
C)
leaf fall and dormancy
done
clear
D)
cell division
done
clear
View Answer play_arrow
question_answer 111) A material, which arrests cell division is obtained from:
A)
Crocus
done
clear
B)
Colchicum
done
clear
C)
Delbergia
done
clear
D)
Chrysanthemum
done
clear
View Answer play_arrow
question_answer 112) African sleeping sickness is caused by:
A)
Trypanosoma cruzi by its vector tse-tse fly
done
clear
B)
T. gambiensis by G. palpalis
done
clear
C)
W. bancroftii by sandfly
done
clear
D)
T. solium by eating measly pork
done
clear
View Answer play_arrow
question_answer 113) Syncytial or coenocytic epidermis is associated with:
A)
Hydra
done
clear
B)
Star fish
done
clear
C)
earthworm
done
clear
D)
Ascaris
done
clear
View Answer play_arrow
question_answer 114) Silver fish is a:
A)
fish
done
clear
B)
crustacean
done
clear
C)
cnidarian
done
clear
D)
insect
done
clear
View Answer play_arrow
question_answer 115) Rhinoceros sp. can be associated with:
A)
Corbett
done
clear
B)
Runn of Kutch
done
clear
C)
Gir
done
clear
D)
Kaziranga
done
clear
View Answer play_arrow
question_answer 116) Soil carried by gravity is:
A)
alluvial
done
clear
B)
colluvial
done
clear
C)
elluvial
done
clear
D)
glacial
done
clear
View Answer play_arrow
question_answer 117) Little leaf disease of brinjal is caused by:
A)
mycoplasma
done
clear
B)
fungus
done
clear
C)
algae
done
clear
D)
virus
done
clear
View Answer play_arrow
question_answer 118) Progesterone is secreted by:
A)
corpus luteum
done
clear
B)
corpus albicans
done
clear
C)
corpus striatum
done
clear
D)
corpus collosum
done
clear
View Answer play_arrow
question_answer 119) Black rust of wheat is caused by:
A)
Rhizopus
done
clear
B)
Puccinia
done
clear
C)
Yeast
done
clear
D)
Penicillium
done
clear
View Answer play_arrow
question_answer 120) Which is not a Coelenterate?
A)
Polyp
done
clear
B)
Portuguese man of war
done
clear
C)
Jellyfish
done
clear
D)
Silver fish
done
clear
View Answer play_arrow
question_answer 121) Pesticides include:
A)
insecticide only
done
clear
B)
fungicides, herbicides, insecticides nematicides and rodenticides
done
clear
C)
insecticides, nematicides and rodenticide
done
clear
D)
herbicides insecticides and nematicides
done
clear
View Answer play_arrow
question_answer 122) A new species is formed when:
A)
because of new genie populations organism with new traits are born
done
clear
B)
exchange of parts between homologous chromosomes takes place
done
clear
C)
change in the genotype in population leads to sexual isolation
done
clear
D)
the exchange of parts of chromosomes during gametogenesis leads to the formation of new genotype
done
clear
View Answer play_arrow
question_answer 123) Which hormone is used to induce ripening in banana?
A)
Cytokinin
done
clear
B)
Ethylene
done
clear
C)
ABA
done
clear
D)
\[G{{A}_{3}}\]
done
clear
View Answer play_arrow
question_answer 124) A new strain for industry produced through biotechnology belongs to;
A)
E. coli
done
clear
B)
Bacillus subtilis
done
clear
C)
Pseudomonas putida
done
clear
D)
Saccharomyces cerevisiae
done
clear
View Answer play_arrow
question_answer 125) Closed ancestor to man was:
A)
Neanderthal man
done
clear
B)
Homo habilis
done
clear
C)
Cro-magnon man
done
clear
D)
Australopithecus
done
clear
View Answer play_arrow
question_answer 126) Prokaryotic DNA is:
A)
double stranded round
done
clear
B)
single stranded round
done
clear
C)
double stranded straight
done
clear
D)
double stranded RNA as nucleic acid
done
clear
View Answer play_arrow
question_answer 127) Klinefelters syndrome is associated with/marked by:
A)
XYY
done
clear
B)
XXY
done
clear
C)
XO
done
clear
D)
XY
done
clear
View Answer play_arrow
question_answer 128) Primary growth is caused by:
A)
apical meristem
done
clear
B)
lateral meristem
done
clear
C)
dermatogen
done
clear
D)
plerome
done
clear
View Answer play_arrow
question_answer 129) Loop of Henie is associated with:
A)
uriniferous tubules
done
clear
B)
seminiferous tubules
done
clear
C)
neurons
done
clear
D)
muscle fibres
done
clear
View Answer play_arrow
question_answer 130) Botanical name of cauliflower is:
A)
Brassica oleracea var. capitata
done
clear
B)
Brassica oleracea var. gemmifera
done
clear
C)
Brassica campestris
done
clear
D)
Brassica oleracea var. botrytis
done
clear
View Answer play_arrow
question_answer 131) Respiratory pigment or oxygen carrier in frogs blood is :
A)
haemocyanin
done
clear
B)
haemoglobin
done
clear
C)
haemazoin
done
clear
D)
lymphocytes
done
clear
View Answer play_arrow
question_answer 132) An introduction to embryology of angiosperm book was written by:
A)
P.Maheshwari
done
clear
B)
K.C. Mehta
done
clear
C)
A.K. Sharma
done
clear
D)
M.S. Swaminathan
done
clear
View Answer play_arrow
question_answer 133) Cardiac muscles contract:
A)
quickly and they fatigue
done
clear
B)
quickly and unfatigued
done
clear
C)
slowly and are not fatigued
done
clear
D)
slowly and they fatigue
done
clear
View Answer play_arrow
question_answer 134) Why Amoeba has been kept in Protozoa?
A)
Due to contractile vacuole
done
clear
B)
Because of nutrition being insectivorous
done
clear
C)
Cell wall
done
clear
D)
Unicellular body
done
clear
View Answer play_arrow
question_answer 135) Alimentary canal is not found in:
A)
Arachnida
done
clear
B)
Apoda
done
clear
C)
Gastropda
done
clear
D)
Cestoda
done
clear
View Answer play_arrow
question_answer 136) Verticillaster, which is the characteristic of family Labiatae is a type of:
A)
phyllotaxis
done
clear
B)
inflorescence
done
clear
C)
placentation
done
clear
D)
venation
done
clear
View Answer play_arrow
question_answer 137) Which one of the following is associated with sex-linked inheritance?
A)
Night-blindness
done
clear
B)
Muscular dystrophy
done
clear
C)
Astigmatism
done
clear
D)
Colourblindness
done
clear
View Answer play_arrow
question_answer 138) The book Micrographia was written by:
A)
Huxley
done
clear
B)
Robert Hooke
done
clear
C)
Fritsch
done
clear
D)
J.D. Hooker
done
clear
View Answer play_arrow
question_answer 139) Velamen tissues are associated with:
A)
haustorial function
done
clear
B)
assimilation
done
clear
C)
absorption of moisture
done
clear
D)
nutrition
done
clear
View Answer play_arrow
question_answer 140) Significance of Krebs cycle:
A)
synthesis of ATP by oxidative phosphorylation
done
clear
B)
synthesis of amino acids
done
clear
C)
synthesis of vitamins
done
clear
D)
activates photosynthesis
done
clear
View Answer play_arrow
question_answer 141) A flower characterised by monoadelphous tubular stamens belongs to :
A)
Solanaceae
done
clear
B)
Liliaceae
done
clear
C)
Malvaceae
done
clear
D)
Brassicaceae
done
clear
View Answer play_arrow
question_answer 142) The upper pigmented part of egg is concerned with :
A)
nourishment
done
clear
B)
camouflage
done
clear
C)
respiration
done
clear
D)
colouration
done
clear
View Answer play_arrow
question_answer 143) Study of relationship between communities and environment is called :
A)
ecology
done
clear
B)
synecology
done
clear
C)
autecology
done
clear
D)
ethology
done
clear
View Answer play_arrow
question_answer 144) The branch of science related with improvement of mankind by genetics is :
A)
human genetics
done
clear
B)
common genetics
done
clear
C)
eugenics
done
clear
D)
heredity
done
clear
View Answer play_arrow
question_answer 145) Biogeochemical cycling means :
A)
cycling of nutrients in an ecosystem
done
clear
B)
cycling of water
done
clear
C)
cycling of energy in an ecosystem
done
clear
D)
cycling of gases between plants and the atmosphere
done
clear
View Answer play_arrow
question_answer 146) Uric acid is the main excretory product in :
A)
insects
done
clear
B)
earthworm
done
clear
C)
amphibians
done
clear
D)
mammals
done
clear
View Answer play_arrow
question_answer 147) In plants, respiration takes place :
A)
during day only
done
clear
B)
during night only
done
clear
C)
all the 24 hours
done
clear
D)
at dusk
done
clear
View Answer play_arrow
question_answer 148) Fossils are :
A)
animals living in burrows
done
clear
B)
remnants of extinct animals and plants
done
clear
C)
floating organisms
done
clear
D)
fast runners
done
clear
View Answer play_arrow
question_answer 149) Semiconservative replication of DNA was given by:
A)
Watson and Crick
done
clear
B)
Bateson and Punnet
done
clear
C)
Messelson and Stahl
done
clear
D)
Avery, McCarty and MacLeod
done
clear
View Answer play_arrow
question_answer 150) Female hormone is :
A)
progesterone
done
clear
B)
estrogen
done
clear
C)
estradiol
done
clear
D)
all of these
done
clear
View Answer play_arrow
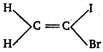
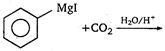
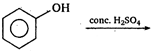
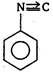
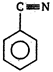
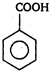
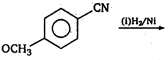 \[(A)\xrightarrow[anhydride]{acetic}(B)\]
\[(A)\xrightarrow[anhydride]{acetic}(B)\]