A) It gives white precipitate with alcoholic \[AgN{{O}_{3}}\]
B) It is an aromatic compound with substitution in the side chain
C) It undergoes nucleophilic substitution reaction
D) It is less reactive than vinyl chloride
Correct Answer: D
Solution :
Benzyl chloride is very reactive. It readily give, white precipitate with alcoholic \[AgN{{O}_{3}}\] at room temperature. It also readily undergo nucleophilic substitution. Its structure is as follows: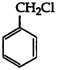 Vinyl chloride \[(C{{H}_{2}}=CH\,.\,Cl)\], on the other hand, is less reactive than benzyl chloride due to resonance.
Vinyl chloride \[(C{{H}_{2}}=CH\,.\,Cl)\], on the other hand, is less reactive than benzyl chloride due to resonance. You need to login to perform this action.
You will be redirected in
3 sec
